Population Abundance and Transience of Selected Coastal Plain Crayfishes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New Alien Crayfish Species in Central Europe
NEW ALIEN CRAYFISH SPECIES IN CENTRAL EUROPE Introduction pathways, life histories, and ecological impacts DISSERTATION zur Erlangung des Doktorgrades Dr. rer. nat. der Fakultät für Naturwissenschaften der Universität Ulm vorgelegt von Christoph Chucholl aus Rosenheim Ulm 2012 NEW ALIEN CRAYFISH SPECIES IN CENTRAL EUROPE Introduction pathways, life histories, and ecological impacts DISSERTATION zur Erlangung des Doktorgrades Dr. rer. nat. der Fakultät für Naturwissenschaften der Universität Ulm vorgelegt von Christoph Chucholl aus Rosenheim Ulm 2012 Amtierender Dekan: Prof. Dr. Axel Groß Erstgutachter: Prof. Dr. Manfred Ayasse Zweitgutachter: Prof. apl. Dr. Gerhard Maier Tag der Prüfung: 16.7.2012 Cover picture: Orconectes immunis male (blue color morph) (photo courtesy of Dr. H. Bellmann) Table of contents Part 1 – Summary Introduction ............................................................................................................................ 1 Invasive alien species – a global menace ....................................................................... 1 “Invasive” matters .......................................................................................................... 2 Crustaceans – successful invaders .................................................................................. 4 The case of alien crayfish in Europe .............................................................................. 5 New versus Old alien crayfish ....................................................................................... -

Species at Risk on Department of Defense Installations
Species at Risk on Department of Defense Installations Revised Report and Documentation Prepared for: Department of Defense U.S. Fish and Wildlife Service Submitted by: January 2004 Species at Risk on Department of Defense Installations: Revised Report and Documentation CONTENTS 1.0 Executive Summary..........................................................................................iii 2.0 Introduction – Project Description................................................................. 1 3.0 Methods ................................................................................................................ 3 3.1 NatureServe Data................................................................................................ 3 3.2 DOD Installations............................................................................................... 5 3.3 Species at Risk .................................................................................................... 6 4.0 Results................................................................................................................... 8 4.1 Nationwide Assessment of Species at Risk on DOD Installations..................... 8 4.2 Assessment of Species at Risk by Military Service.......................................... 13 4.3 Assessment of Species at Risk on Installations ................................................ 15 5.0 Conclusion and Management Recommendations.................................... 22 6.0 Future Directions............................................................................................. -

Louisiana's Animal Species of Greatest Conservation Need (SGCN)
Louisiana's Animal Species of Greatest Conservation Need (SGCN) ‐ Rare, Threatened, and Endangered Animals ‐ 2020 MOLLUSKS Common Name Scientific Name G‐Rank S‐Rank Federal Status State Status Mucket Actinonaias ligamentina G5 S1 Rayed Creekshell Anodontoides radiatus G3 S2 Western Fanshell Cyprogenia aberti G2G3Q SH Butterfly Ellipsaria lineolata G4G5 S1 Elephant‐ear Elliptio crassidens G5 S3 Spike Elliptio dilatata G5 S2S3 Texas Pigtoe Fusconaia askewi G2G3 S3 Ebonyshell Fusconaia ebena G4G5 S3 Round Pearlshell Glebula rotundata G4G5 S4 Pink Mucket Lampsilis abrupta G2 S1 Endangered Endangered Plain Pocketbook Lampsilis cardium G5 S1 Southern Pocketbook Lampsilis ornata G5 S3 Sandbank Pocketbook Lampsilis satura G2 S2 Fatmucket Lampsilis siliquoidea G5 S2 White Heelsplitter Lasmigona complanata G5 S1 Black Sandshell Ligumia recta G4G5 S1 Louisiana Pearlshell Margaritifera hembeli G1 S1 Threatened Threatened Southern Hickorynut Obovaria jacksoniana G2 S1S2 Hickorynut Obovaria olivaria G4 S1 Alabama Hickorynut Obovaria unicolor G3 S1 Mississippi Pigtoe Pleurobema beadleianum G3 S2 Louisiana Pigtoe Pleurobema riddellii G1G2 S1S2 Pyramid Pigtoe Pleurobema rubrum G2G3 S2 Texas Heelsplitter Potamilus amphichaenus G1G2 SH Fat Pocketbook Potamilus capax G2 S1 Endangered Endangered Inflated Heelsplitter Potamilus inflatus G1G2Q S1 Threatened Threatened Ouachita Kidneyshell Ptychobranchus occidentalis G3G4 S1 Rabbitsfoot Quadrula cylindrica G3G4 S1 Threatened Threatened Monkeyface Quadrula metanevra G4 S1 Southern Creekmussel Strophitus subvexus -

US Fish & Wildlife Service
U.S. FishU.S &. FishWildlife & Wildlife Service Service Kisatchie Painted Crayfish Arlington, Texas Ecological Services Field Office Kisatchie Painted Crayfish Faxonius maletae During the winter, the mortality rate of crayfish Description increases due to mating stress, starvation, and The Kisatchie painted crayfish predation. Abiotic (Faxonius maletae) is an elusive, factors such as low freshwater crayfish endemic to the dissolved oxygen levels streams of northeast Texas and and temperature Louisiana. It is characterized by an fluctuations can also olive carapace (hard, upper shell) influence crayfish and the red marks on the chelae survival. (claws), legs, and above the eyes. Crayfish, in general, are keystone species that may indicate the health F. maletae Habitat of a watershed, and nearly half of Photo: Steve Shively, USDA Forest Service Little is known about the crayfish species are vulnerable, habitat requirements of the determined that the Kisatchie painted threatened, or endangered. The size of Kisatchie painted crayfish. They were crayfish was absent from 60% of its Kisatchie painted crayfish appears to historically collected in freshwater historical range in Texas. be influenced by water depth. streams with sand, gravel, mud, or silt Individuals found in deep water have in northeast Texas and Louisiana. been documented to reach lengths of Life History However, the Texas habitat was more 101.6 mm, while those found in The Kisatchie painted crayfish is stagnant and muddy than Louisiana. shallow water rarely reach lengths reproductively active in September Kisatchie painted crayfish may prefer over 50.8 mm. and October; unlike other crayfish that streams with varying water depth, reproduce in the spring. -

Biological Evaluation Usda - Forest Service, Kisatchie National Forest Catahoula Ranger District
Catahoula RD – North Gray Creek 2 Aug 19 BIOLOGICAL EVALUATION USDA - FOREST SERVICE, KISATCHIE NATIONAL FOREST CATAHOULA RANGER DISTRICT North Gray Creek I. INTRODUCTION This report documents the findings of the Biological Evaluation (BE) for the proposed silvicultural activities in Compartments 89-93 on the Catahoula Ranger District of the Kisatchie National Forest. It also serves to provide the decision maker with information and determinations of the effects of proposed actions on proposed, endangered, threatened and sensitive (PETS) species and habitats so that the best decisions can be made regarding these species and the proposal. PETS species are species whose viability is most likely to be put at risk from management actions. Through the BE process the proposed management activities were reviewed and their potential effects on PETS species disclosed. Evaluation methods included internal expertise on species' habitat requirements, field surveys, Forest Service inventory and occurrence records, Final Environmental Impact Statement/Revised Land and Resource Management Plan for the Kisatchie National Forest, the recovery plans for the the red- cockaded woodpecker (RCW) and Louisiana pearlshell mussel (LPM) and the draft recovery plan and candidate conservation agreement (CCA) for the Louisisana pine snake (LPS). This biological evaluation was prepared in accordance with Forest Service Handbook 2609.23R and regulations set forth in Section 7 (a)(2) of the Endangered Species Act. A botanical evaluation was done separately to address impacts on sensitive and conservation plants. PURPOSE AND NEED: Differences between current and desired conditions have been identified within the project area. In order to move the project area toward the desired conditions, specific resource management actions were identified and developed. -

The Status of the Kisatchie Painted Crayfish (Faxonius Maletae) in Louisiana
University of Texas at Tyler Scholar Works at UT Tyler Biology Theses Biology Spring 3-22-2019 The tS atus of the Kisatchie Painted Crayfish (Faxonius maletae) in Louisiana Jade L.M. McCarley Follow this and additional works at: https://scholarworks.uttyler.edu/biology_grad Part of the Biology Commons Recommended Citation McCarley, Jade L.M., "The tS atus of the Kisatchie Painted Crayfish (Faxonius maletae) in Louisiana" (2019). Biology Theses. Paper 58. http://hdl.handle.net/10950/1317 This Thesis is brought to you for free and open access by the Biology at Scholar Works at UT Tyler. It has been accepted for inclusion in Biology Theses by an authorized administrator of Scholar Works at UT Tyler. For more information, please contact [email protected]. THE STATUS OF THE KISATCHIE PAINTED CRAYFISH (FAXONIUS MALETAE) IN LOUISIANA by JADE L. M. MCCARLEY A thesis submitted in the partial fulfillment of the requirements for the degree of Master of Biology Department of Biology Lance R. Williams, Ph.D., Committee Chair College of Arts and Sciences The University of Texas at Tyler May 2019 © Copyright Jade L. M. McCarley 2019 All rights reserved ACKNOWLEDGMENTS I would like to acknowledge Dr. Bob Wagner, Quantitative Ecological Services (QES), Jody Patterson, Sarah Pearce, and the staff at Fort Polk for all of their support throughout this project. I am sincerely grateful for my advisor, Lance Williams for this opportunity. He has guided me through graduate school and has inspired me to continue as a scientist. Thank you, Marsha Williams for your dedication to field sampling and especially for welcoming me into your family while at UT. -

Southern White River Crawfish Procambarus Zonangulus
Southern White River Crawfish Procambarus zonangulus Identification These crayfish have a space called an areola separating the sides of the back, forming a gap in the middle. Color is usually brown, with pink or purple in some adults. Mature crawfish have more elongated and cylindrical claws. Usually have white or tan walking legs. Why is it a Like other non-native crayfish, Problem? this species competes with and displaces native crayfish Left: Red swamp crayfish; species. It also reduces the Right: Southern white river crawfish abundance and diversity of aquatic life. Range/Habitat Native range is southeastern Want to know more? Check out Texas, Alabama, Louisiana, and www.dnr.maryland.gov for more on Mississippi. Introduced to other invasive species in Maryland and states including Maryland and what you can do about it. West Virginia. Similar Species White river crawfish (Procambarus acutus actus); Method of red swamp crayfish Introduction Established in Maryland as a (Procambarus clarkii) result of aquaculture. Control and Prevention Do not release live, unused Legal Status bait. Only use bait at site of capture. Do not transport live crayfish from one body of water to another. Sources: Jay V. Kilian, Andrew J. Becker, Scott A. Stranko, Matthew Ashton, Ronald J. Klauda, Jay Gerber & Martin Hurd (2010). "The Status and Distribution of Maryland Crayfishes". Southeastern Naturalist 9 (sp3): 11–32. "Crayfish in Alabama". Alabama Department of Conservation and Natural Resources. 2008. http://www.outdooralabama.com/watchable-wildlife/what/inverts/crayfish/. http://www.rw.ttu.edu/patino/Teaching/Aquaculture/PowerPoints/Lec%2018_Freshwater%20crustaceans.ppt#2 6 http://www.lsuagcenter.com/en/our_offices/research_stations/Aquaculture/Features/extension/Classroom_Reso urces/The+Difference+Between+Red+Swamp+Crawfish+and+White+River+Crawfish.htm . -

Crawfish Economics Teacher Instructions
Crawfish Economics Teacher Instructions Overview: Grade Levels: The crawfish industry is an important part of Louisiana’s economy. Upper elementary The red swamp crawfish is one of the crawfish species used for widespread sale and consumption. In this lesson, students will Subject Area: learn how Louisiana’s native red swamp crawfish have been Social Studies transplanted to other countries around the world and have thrived. Chinese crawfish even have become a competitor in the state. Duration: One class period Learning Objectives: The students will: Setting: Understand how the red swamp crawfish traveled from Classroom Louisiana to Central America to Europe to Africa and to Asia. Vocabulary: Explain how the red swamp crawfish became a part of Circumnavigate China’s export business. Consumers Determine the positive and negative consequences for Crop rotation Louisiana’s red swamp crawfish industry because of other Crustacean countries’ red swamp crawfish industries. Demand Economy Materials List: Native species Globe (teacher provides) Introduced species Crayons Natural resource Scissors Producer Renewable natural Grade Level Expectations: resource Third Grade Supply 16. Identify and compare customs, celebrations and traditions of various cultural groups in Louisiana. (G-1C-E4) 21. Identify natural resources in Louisiana and describe their uses and importance. (G- 1D-E4) 37. Identify the concepts of specialization (i.e., being an expert in one job, product, or service) and interdependence (i.e., depending on others) in the production of goods and services. (E-1A-E7) 39. Identify goods that are produced within the local community and Louisiana and describe how they are shipped elsewhere for sale. (E-1A-E9) 42. -

Rare Animals Tracking List
Louisiana's Animal Species of Greatest Conservation Need (SGCN) ‐ Rare, Threatened, and Endangered Animals ‐ 2020 MOLLUSKS Common Name Scientific Name G‐Rank S‐Rank Federal Status State Status Mucket Actinonaias ligamentina G5 S1 Rayed Creekshell Anodontoides radiatus G3 S2 Western Fanshell Cyprogenia aberti G2G3Q SH Butterfly Ellipsaria lineolata G4G5 S1 Elephant‐ear Elliptio crassidens G5 S3 Spike Elliptio dilatata G5 S2S3 Texas Pigtoe Fusconaia askewi G2G3 S3 Ebonyshell Fusconaia ebena G4G5 S3 Round Pearlshell Glebula rotundata G4G5 S4 Pink Mucket Lampsilis abrupta G2 S1 Endangered Endangered Plain Pocketbook Lampsilis cardium G5 S1 Southern Pocketbook Lampsilis ornata G5 S3 Sandbank Pocketbook Lampsilis satura G2 S2 Fatmucket Lampsilis siliquoidea G5 S2 White Heelsplitter Lasmigona complanata G5 S1 Black Sandshell Ligumia recta G4G5 S1 Louisiana Pearlshell Margaritifera hembeli G1 S1 Threatened Threatened Southern Hickorynut Obovaria jacksoniana G2 S1S2 Hickorynut Obovaria olivaria G4 S1 Alabama Hickorynut Obovaria unicolor G3 S1 Mississippi Pigtoe Pleurobema beadleianum G3 S2 Louisiana Pigtoe Pleurobema riddellii G1G2 S1S2 Pyramid Pigtoe Pleurobema rubrum G2G3 S2 Texas Heelsplitter Potamilus amphichaenus G1G2 SH Fat Pocketbook Potamilus capax G2 S1 Endangered Endangered Inflated Heelsplitter Potamilus inflatus G1G2Q S1 Threatened Threatened Ouachita Kidneyshell Ptychobranchus occidentalis G3G4 S1 Rabbitsfoot Quadrula cylindrica G3G4 S1 Threatened Threatened Monkeyface Quadrula metanevra G4 S1 Southern Creekmussel Strophitus subvexus -
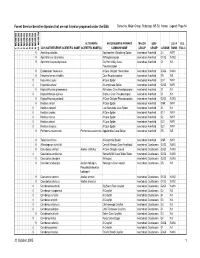
Sensitive Species That Are Not Listed Or Proposed Under the ESA Sorted By: Major Group, Subgroup, NS Sci
Forest Service Sensitive Species that are not listed or proposed under the ESA Sorted by: Major Group, Subgroup, NS Sci. Name; Legend: Page 94 REGION 10 REGION 1 REGION 2 REGION 3 REGION 4 REGION 5 REGION 6 REGION 8 REGION 9 ALTERNATE NATURESERVE PRIMARY MAJOR SUB- U.S. N U.S. 2005 NATURESERVE SCIENTIFIC NAME SCIENTIFIC NAME(S) COMMON NAME GROUP GROUP G RANK RANK ESA C 9 Anahita punctulata Southeastern Wandering Spider Invertebrate Arachnid G4 NNR 9 Apochthonius indianensis A Pseudoscorpion Invertebrate Arachnid G1G2 N1N2 9 Apochthonius paucispinosus Dry Fork Valley Cave Invertebrate Arachnid G1 N1 Pseudoscorpion 9 Erebomaster flavescens A Cave Obligate Harvestman Invertebrate Arachnid G3G4 N3N4 9 Hesperochernes mirabilis Cave Psuedoscorpion Invertebrate Arachnid G5 N5 8 Hypochilus coylei A Cave Spider Invertebrate Arachnid G3? NNR 8 Hypochilus sheari A Lampshade Spider Invertebrate Arachnid G2G3 NNR 9 Kleptochthonius griseomanus An Indiana Cave Pseudoscorpion Invertebrate Arachnid G1 N1 8 Kleptochthonius orpheus Orpheus Cave Pseudoscorpion Invertebrate Arachnid G1 N1 9 Kleptochthonius packardi A Cave Obligate Pseudoscorpion Invertebrate Arachnid G2G3 N2N3 9 Nesticus carteri A Cave Spider Invertebrate Arachnid GNR NNR 8 Nesticus cooperi Lost Nantahala Cave Spider Invertebrate Arachnid G1 N1 8 Nesticus crosbyi A Cave Spider Invertebrate Arachnid G1? NNR 8 Nesticus mimus A Cave Spider Invertebrate Arachnid G2 NNR 8 Nesticus sheari A Cave Spider Invertebrate Arachnid G2? NNR 8 Nesticus silvanus A Cave Spider Invertebrate Arachnid G2? NNR -

BIOLOGICAL EVALUATION (Conservation Species) USDA FOREST SERVICE Kisatchie National Forest Winn Ranger District
BIOLOGICAL EVALUATION (Conservation Species) USDA FOREST SERVICE Kisatchie National Forest Winn Ranger District Mendenhause Project Compartments 51, 52, and 56 INTRODUCTION: The purpose of this Biological Evaluation (BE) is to ensure that Forest Service actions do not contribute to the loss of viability of any native or desirable non-native animal species. This BE documents likely effects of management actions on populations of conservation species of concern as determined by the Louisiana Natural Heritage Program (LNHP). Such species are those, whose viability is most likely to be put at risk from management actions. Information presented here is used to ensure that such species are maintained at, or are moving toward, viable population levels. Populations of other species (those at less risk of losing viability) are maintained by creating and maintaining a diversity of habitat types distributed across the National Forest in accordance with Forest Plan Standards and Guidelines. Through this combination of approaches, viable populations of all species are maintained. A review of the Louisiana Rare Animal Species list and the Forest Geographic Information System (GIS) records was conducted to determine which species would possibly occur within the project site. The general biology of the species, personal communication with experts, and literature searches were used to determine the potential effect proposed actions, including the no action, would have on species considered likely to occur within the project area. The aforementioned research was conducted considering the best available science, and the potential effects discovered are discussed in this BE. PURPOSE AND NEED: Differences between current and desired conditions have been identified within the project area. -

White River Crayfish Procambarusprocambarus Acutus Aacutushemimysis Acutus Acutus Anomala
White River Crayfish www.seagrant.psu.edu ProcambarusProcambarus acutus aacutusHemimysis acutus acutus anomala The white river crayfish, also called the white river crawfish and the eastern white river crayfish, is often confused with its southern counterpart, the southern white river crayfish (Procambarus zonangulus). The eastern white river crayfish occurs naturally in the United States and is cultured eastwards from Lousiana to the Atlantic coast northward to Maine, Photo courtesy of Tony Palacios, but has established select non-native populations in locations throughout the East Coast iNaturalist.org, EOL. and in California. Species Description Adult white river crayfish are usually a dark burgundy red but can range in color from pinkish tan to brownish olive with a black “V-shaped” stripe on the abdomen. The carapace is rough and granular and is separated in the middle by a narrow space called the areola. Juveniles are gray with dark spots scattered over the carapace. The claws are long and narrow, delicate in appearance, and have small dark tubercles. This species reaches about 6-13 cm (2.5-5 in) in length. The white river crayfish is nearly impossible to distinguish from the southern white river crayfish without looking at the reproductive structures of a breeding male. It is also confused with the red swamp crayfish (P. clarkii), which have an areola that is straight, or often invisible, and a black “V-shaped” stripe on the abdomen. The juveniles are also typically plain or striped on the carapace instead of spotted. White river crayfish can also be found in streams and ditches with a stronger flow than what is preferred by the red swamp crayfish.