Generate Metabolic Map Poster
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

One Carbon Metabolism and Its Clinical Significance
One carbon metabolism and its clinical significance Dr. Kiran Meena Department of Biochemistry Class 5 : 10-10-2019 (8:00 to 9:00 AM ) Specific Learning Objectives Describe roles of folic acid, cobalamin and S-adenosylmethionine (SAM) in transfer of one carbon units between molecules, and apply their relevance to disease states Describe synthesis of S-adenosylmethionine and its role in methylation reactions Explain how a cobalamin deficiency leads to a secondary folate deficiency Introduction Human body cannot synthesize folic acid Its also called vitamin B9 give rise to tetrahydrofolate (THF), carry one carbon groups ex. Methyl group Intestines releases mostly N5-methy-THF into blood One-carbon (1C) metabolism, mediated by folate cofactor, supports biosynthesis of purines and pyrimidines, aa homeostasis (glycine, serine and methionine) Table 14.4: DM Vasudevan’ s Textbook of Biochemistry for Medical Students 6th edition Enzyme co-factors involved in aa catabolism Involves one of three co-factors: Biotin, Tetrahydrofolate (THF) and S- adenosylmethionine (SAM) These cofactors transfer one-carbon groups in different oxidation states: 1. Biotin transfers carbon in its most oxidized state CO2, it require for catabolism and utilization of branched chain aa • Biotin responsible for carbon dioxide transfer in several carboxylase enzymes Cont-- 2. Tetrahydrofolate (THF) transfers one-carbon groups in intermediate oxidation states and as methyl groups • Tetrahydrobiopterin (BH4, THB) is a cofactor of degradation of phenylalanine • Oxidised form of THF, folate is vitamin for mammals • It converted into THF by DHF reductase 3. S-adenosylmethionine (SAM) transfers methyl groups, most reduced state of carbon THF and SAM imp in aa and nucleotide metabolism SAM used in biosynthesis of creatine, phosphatidylcholine, plasmenylcholine and epinephrine, also methylated DNA, RNA and proteins Enzymes use cobalamin as a cofactor 1. -

Reduction by the Methylreductase System in Methanobacterium Bryantii WILLIAM B
JOURNAL OF BACTERIOLOGY, Jan. 1987, p. 87-92 Vol. 169, No. 1 0021-9193/87/010087-06$02.00/0 Copyright © 1987, American Society for Microbiology Inhibition by Corrins of the ATP-Dependent Activation and CO2 Reduction by the Methylreductase System in Methanobacterium bryantii WILLIAM B. WHITMAN'* AND RALPH S. WOLFE2 Department of Microbiology, University of Georgia, Athens, Georgia 30602,1 and Department of Microbiology, University ofIllinois, Urbana, Illinois 618012 Received 1 August 1986/Accepted 28 September 1986 Corrins inhibited the ATP-dependent activation of the methylreductase system and the methyl coenzyme M-dependent reduction of CO2 in extracts of Methanobacterium bryantii resolved from low-molecular-weight factors. The concentrations of cobinamides and cobamides required for one-half of maximal inhibition of the ATP-depen4ent activation were between 1 and 5 ,M. Cobinamides were more inhibitory at lower concentra- tiops than cobamides. Deoxyadenosylcobalamin was not inhibitory at concentrations up to 25 ,uM. The inhibition of CO2 reduction was competitive with respect to CO2. The concentration of methylcobalamin required for one-half of maximal inhibition was 5 ,M. Other cobamideg inhibited at similar concentrations, but diaquacobinami4e inhibited at lower concentrations. With respect to their affinities and specificities for corrins, inhibition of both the ATP-dependent activation'and CO2 reduction closely resembled the corrin- dependent activation of the methylreductase described in similar extracts (W. B. Whitman and R. S. Wolfe, J. Bacteriol. 164:165-172, 1985). However, whether the multiple effects of corrins are due to action at a single site is unknown. The effect of corrins (cobamides and cobinamides) on in CO2 reduction. -

On the Active Site Thiol of Y-Glutamylcysteine Synthetase
Proc. Natl. Acad. Sci. USA Vol. 85, pp. 2464-2468, April 1988 Biochemistry On the active site thiol of y-glutamylcysteine synthetase: Relationships to catalysis, inhibition, and regulation (glutathione/cystamine/Escherichia coli/kidney/enzyme inactivation) CHIN-SHIou HUANG, WILLIAM R. MOORE, AND ALTON MEISTER Cornell University Medical College, Department of Biochemistry, 1300 York Avenue, New York, NY 10021 Contributed by Alton Meister, December 4, 1987 ABSTRACT y-Glutamylcysteine synthetase (glutamate- dithiothreitol, suggesting that cystamine forms a mixed cysteine ligase; EC 6.3.2.2) was isolated from an Escherichia disulfide between cysteamine and an enzyme thiol (15). coli strain enriched in the gene for this enzyme by recombinant Inactivation of the enzyme by the L- and D-isomers of DNA techniques. The purified enzyme has a specific activity of 3-amino-1-chloro-2-pentanone, as well as that by cystamine, 1860 units/mg and a molecular weight of 56,000. Comparison is prevented by L-glutamate (14). Treatment of the enzyme of the E. coli enzyme with the well-characterized rat kidney with cystamine prevents its interaction with the sulfoxi- enzyme showed that these enzymes have similar catalytic prop- mines. Titration of the enzyme with 5,5'-dithiobis(2- erties (apparent Km values, substrate specificities, turnover nitrobenzoate) reveals that the enzyme has a single exposed numbers). Both enzymes are feedback-inhibited by glutathione thiol that reacts with this reagent without affecting activity but not by y-glutamyl-a-aminobutyrylglycine; the data indicate (16). 5,5'-Dithiobis(2-nitrobenzoate) does not interact with that glutathione binds not only at the glutamate binding site but the thiol that reacts with cystamine. -

Methionine Sulfoximine: a Novel Anti Inflammatory Agent
Wayne State University Wayne State University Dissertations January 2018 Methionine Sulfoximine: A Novel Anti Inflammatory Agent Tyler Peters Wayne State University, [email protected] Follow this and additional works at: https://digitalcommons.wayne.edu/oa_dissertations Part of the Biochemistry Commons Recommended Citation Peters, Tyler, "Methionine Sulfoximine: A Novel Anti Inflammatory Agent" (2018). Wayne State University Dissertations. 2124. https://digitalcommons.wayne.edu/oa_dissertations/2124 This Open Access Dissertation is brought to you for free and open access by DigitalCommons@WayneState. It has been accepted for inclusion in Wayne State University Dissertations by an authorized administrator of DigitalCommons@WayneState. METHIONINE SULFOXIMINE: A NOVEL ANTI-INFLAMMATORY AGENT by TYLER J. PETERS DISSERTATION Submitted to the Graduate School of Wayne State University – School of Medicine Detroit, Michigan in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOHPY 2018 MAJOR: BIOCHEMISTRY & MOL. BIOLOGY Approved By: __________________________________________ Advisor Date DEDICATION This work is dedicated to my family. I wouldn’t have made it this far without your unconditional love and support. ii ACKNOWLEDGEMENTS Thank you Dr. Brusilow, I consider myself very fortunate for having the privilege of working in the laboratory of Dr. William S.A. Brusilow these past few years. Under his mentorship, my scientific autonomy was always respected, and my opinions were always valued with consideration. I am thankful for his guidance and support as an advisor; I truly admire his patience and envy his calm demeanor. He exemplifies scientific integrity, and his dedication to develop MSO has inspired me. I had never experienced consistent failure in any aspect of life before encountering scientific research; at times I felt that Dr. -

Mobile Loop Dynamics in Adenosyltransferase Control
Mobile loop dynamics in adenosyltransferase control binding and reactivity of coenzyme B12 Romila Mascarenhasa,1, Markus Ruetza,1, Liam McDevitta, Markos Koutmosb,c, and Ruma Banerjeea,2 aDepartment of Biological Chemistry, University of Michigan, Ann Arbor, MI 48109-0600; bDepartment of Chemistry, University of Michigan, Ann Arbor, MI 48109-0600, and cDepartment of Biophysics, University of Michigan, Ann Arbor, MI 48109-0600 Edited by Amie K. Boal, Pennsylvania State University, State College, PA, and accepted by Editorial Board Member Stephen J. Benkovic October 20, 2020 (received for review April 16, 2020) Cobalamin is a complex organometallic cofactor that is processed Human and Methylobacterium extorquens (Me) ATR, which have and targeted via a network of chaperones to its dependent been characterized most extensively, also double as chaperones, enzymes. AdoCbl (5′-deoxyadenosylcobalamin) is synthesized transferring AdoCbl directly to MCM (Fig. 1A) rather than re- from cob(II)alamin in a reductive adenosylation reaction catalyzed leasing the high-value product into solution (12–17). Cofactor by adenosyltransferase (ATR), which also serves as an escort, de- loading onto MCM is gated by the GTPase activity of the G livering AdoCbl to methylmalonyl-CoA mutase (MCM). The mech- protein chaperone CblA (18). Despite the overall structural and anism by which ATR signals that its cofactor cargo is ready functional conservation of the cofactor loading processes, there (AdoCbl) or not [cob(II)alamin] for transfer to MCM, is not known. are important differences in regulation between the human (19) In this study, we have obtained crystallographic snapshots that – reveal ligand-induced ordering of the N terminus of Mycobacte- and the better-characterized bacterial systems (15 17). -
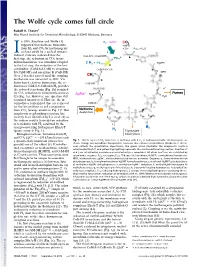
The Wolfe Cycle Comes Full Circle
The Wolfe cycle comes full circle Rudolf K. Thauer1 Max Planck Institute for Terrestrial Microbiology, D-35043 Marburg, Germany n 1988, Rouvière and Wolfe (1) H - ΔμNa+ 2 CO2 suggested that methane formation + MFR from H and CO by methanogenic + 2H+ *Fd + H O I 2 2 ox 2 archaea could be a cyclical process. j O = Indirect evidence indicated that the CoB-SH + CoM-SH fi *Fd 2- a rst step, the reduction of CO2 to for- red R mylmethanofuran, was somehow coupled + * H MPT 2 H2 Fdox 4 to the last step, the reduction of the het- h erodisulfide (CoM-S-S-CoB) to coenzyme CoM-S-S-CoB b MFR M (CoM-SH) and coenzyme B (CoB-SH). H Over 2 decades passed until the coupling C 4 10 mechanism was unraveled in 2011: Via g flavin-based electron bifurcation, the re- CoB-SH duction of CoM-S-S-CoB with H provides 2 H+ the reduced ferredoxin (Fig. 1h) required c + Purines for CO2 reduction to formylmethanofuran ΔμNa + H MPT 4 f H O (2) (Fig. 1a). However, one question still 2 remained unanswered: How are the in- termediates replenished that are removed CoM-SH for the biosynthesis of cell components H Methionine d from CO2 (orange arrows in Fig. 1)? This Acetyl-CoA e anaplerotic (replenishing) reaction has F420 F420H2 recently been identified by Lie et al. (3) as F420 F420H2 the sodium motive force-driven reduction H i of ferredoxin with H2 catalyzed by the i energy-converting hydrogenase EhaA-T H2 (green arrow in Fig. -

Characterization of the Scavenger Cell Proteome in Mouse and Rat Liver
Biol. Chem. 2021; 402(9): 1073–1085 Martha Paluschinski, Cheng Jun Jin, Natalia Qvartskhava, Boris Görg, Marianne Wammers, Judith Lang, Karl Lang, Gereon Poschmann, Kai Stühler and Dieter Häussinger* Characterization of the scavenger cell proteome in mouse and rat liver + https://doi.org/10.1515/hsz-2021-0123 The data suggest that the population of perivenous GS Received January 25, 2021; accepted July 4, 2021; scavenger cells is heterogeneous and not uniform as previ- published online July 30, 2021 ously suggested which may reflect a functional heterogeneity, possibly relevant for liver regeneration. Abstract: The structural-functional organization of ammonia and glutamine metabolism in the liver acinus involves highly Keywords: glutaminase; glutamine synthetase; liver specialized hepatocyte subpopulations like glutamine syn- zonation; proteomics; scavenger cells. thetase (GS) expressing perivenous hepatocytes (scavenger cells). However, this cell population has not yet been char- acterized extensively regarding expression of other genes and Introduction potential subpopulations. This was investigated in the present study by proteome profiling of periportal GS-negative and There is a sophisticated structural-functional organization in perivenous GS-expressing hepatocytes from mouse and rat. the liver acinus with regard to ammonium and glutamine Apart from established markers of GS+ hepatocytes such as metabolism (Frieg et al. 2021; Gebhardt and Mecke 1983; glutamate/aspartate transporter II (GLT1) or ammonium Häussinger 1983, 1990). Periportal hepatocytes express en- transporter Rh type B (RhBG), we identified novel scavenger zymes required for urea synthesis such as the rate-controlling cell-specific proteins like basal transcription factor 3 (BTF3) enzyme carbamoylphosphate synthetase 1 (CPS1) and liver- and heat-shock protein 25 (HSP25). -

Resolution of Carbon Metabolism and Sulfur-Oxidation Pathways of Metallosphaera Cuprina Ar-4 Via Comparative Proteomics
JOURNAL OF PROTEOMICS 109 (2014) 276– 289 Available online at www.sciencedirect.com ScienceDirect www.elsevier.com/locate/jprot Resolution of carbon metabolism and sulfur-oxidation pathways of Metallosphaera cuprina Ar-4 via comparative proteomics Cheng-Ying Jianga, Li-Jun Liua, Xu Guoa, Xiao-Yan Youa, Shuang-Jiang Liua,c,⁎, Ansgar Poetschb,⁎⁎ aState Key Laboratory of Microbial Resources, Institute of Microbiology, Chinese Academy of Sciences, Beijing, PR China bPlant Biochemistry, Ruhr University Bochum, Bochum, Germany cEnvrionmental Microbiology and Biotechnology Research Center, Institute of Microbiology, Chinese Academy of Sciences, Beijing, PR China ARTICLE INFO ABSTRACT Article history: Metallosphaera cuprina is able to grow either heterotrophically on organics or autotrophically Received 16 March 2014 on CO2 with reduced sulfur compounds as electron donor. These traits endowed the species Accepted 6 July 2014 desirable for application in biomining. In order to obtain a global overview of physiological Available online 14 July 2014 adaptations on the proteome level, proteomes of cytoplasmic and membrane fractions from cells grown autotrophically on CO2 plus sulfur or heterotrophically on yeast extract Keywords: were compared. 169 proteins were found to change their abundance depending on growth Quantitative proteomics condition. The proteins with increased abundance under autotrophic growth displayed Bioleaching candidate enzymes/proteins of M. cuprina for fixing CO2 through the previously identified Autotrophy 3-hydroxypropionate/4-hydroxybutyrate cycle and for oxidizing elemental sulfur as energy Heterotrophy source. The main enzymes/proteins involved in semi- and non-phosphorylating Entner– Industrial microbiology Doudoroff (ED) pathway and TCA cycle were less abundant under autotrophic growth. Also Extremophile some transporter proteins and proteins of amino acid metabolism changed their abundances, suggesting pivotal roles for growth under the respective conditions. -

Supplemental Methods
Supplemental Methods: Sample Collection Duplicate surface samples were collected from the Amazon River plume aboard the R/V Knorr in June 2010 (4 52.71’N, 51 21.59’W) during a period of high river discharge. The collection site (Station 10, 4° 52.71’N, 51° 21.59’W; S = 21.0; T = 29.6°C), located ~ 500 Km to the north of the Amazon River mouth, was characterized by the presence of coastal diatoms in the top 8 m of the water column. Sampling was conducted between 0700 and 0900 local time by gently impeller pumping (modified Rule 1800 submersible sump pump) surface water through 10 m of tygon tubing (3 cm) to the ship's deck where it then flowed through a 156 µm mesh into 20 L carboys. In the lab, cells were partitioned into two size fractions by sequential filtration (using a Masterflex peristaltic pump) of the pre-filtered seawater through a 2.0 µm pore-size, 142 mm diameter polycarbonate (PCTE) membrane filter (Sterlitech Corporation, Kent, CWA) and a 0.22 µm pore-size, 142 mm diameter Supor membrane filter (Pall, Port Washington, NY). Metagenomic and non-selective metatranscriptomic analyses were conducted on both pore-size filters; poly(A)-selected (eukaryote-dominated) metatranscriptomic analyses were conducted only on the larger pore-size filter (2.0 µm pore-size). All filters were immediately submerged in RNAlater (Applied Biosystems, Austin, TX) in sterile 50 mL conical tubes, incubated at room temperature overnight and then stored at -80oC until extraction. Filtration and stabilization of each sample was completed within 30 min of water collection. -

Coordinated Slowing of Metabolism in Enteric Bacteria Under Nitrogen
Coordinated Slowing of Metabolism in Enteric Bacteria under Nitrogen Limitation: A Perspective Ned S. Wingreen NEC Research Institute, 4 Independence Way Princeton, New Jersey 08540 and Department of Physics, University of California Berkeley, CA 94720 Sydney Kustu Department of Plant Biology, Molecular and Cell Biology University of California, Berkeley, CA 94720 Abstract It is natural to ask how bacteria coordinate metabolism when depletion of an essential nutrient limits their growth, and they must slow their entire rate of biosyn- thesis. A major nutrient with a fluctuating abundance is nitrogen. The growth rate of enteric bacteria under nitrogen-limiting conditions is known to correlate with the internal concentration of free glutamine, the glutamine pool. Here we compare the patterns of utilization of L-glutamine and L-glutamate, the two central inter- mediates of nitrogen metabolism. Monomeric precursors of all of the cell’s macro- molecules – proteins, nucleic acids, and surface polymers – require the amide group of glutamine at the first dedicated step of biosynthesis. This is the case even though only a minority (∼12%) of total cell nitrogen derives from glutamine. In contrast, the amino group of glutamate, which provides the remainder of cell nitrogen, is arXiv:physics/0110037v1 [physics.bio-ph] 12 Oct 2001 generally required late in biosynthetic pathways, e.g. in transaminase reactions for amino acid synthesis. We propose that the pattern of glutamine dependence coor- dinates the decrease in biosynthesis under conditions of nitrogen limitation. Hence, the glutamine pool plays a global regulatory role in the cell. 1 INTRODUCTION Enteric bacteria are notable for their varying environment. -

Letters to Nature
letters to nature Received 7 July; accepted 21 September 1998. 26. Tronrud, D. E. Conjugate-direction minimization: an improved method for the re®nement of macromolecules. Acta Crystallogr. A 48, 912±916 (1992). 1. Dalbey, R. E., Lively, M. O., Bron, S. & van Dijl, J. M. The chemistry and enzymology of the type 1 27. Wolfe, P. B., Wickner, W. & Goodman, J. M. Sequence of the leader peptidase gene of Escherichia coli signal peptidases. Protein Sci. 6, 1129±1138 (1997). and the orientation of leader peptidase in the bacterial envelope. J. Biol. Chem. 258, 12073±12080 2. Kuo, D. W. et al. Escherichia coli leader peptidase: production of an active form lacking a requirement (1983). for detergent and development of peptide substrates. Arch. Biochem. Biophys. 303, 274±280 (1993). 28. Kraulis, P.G. Molscript: a program to produce both detailed and schematic plots of protein structures. 3. Tschantz, W. R. et al. Characterization of a soluble, catalytically active form of Escherichia coli leader J. Appl. Crystallogr. 24, 946±950 (1991). peptidase: requirement of detergent or phospholipid for optimal activity. Biochemistry 34, 3935±3941 29. Nicholls, A., Sharp, K. A. & Honig, B. Protein folding and association: insights from the interfacial and (1995). the thermodynamic properties of hydrocarbons. Proteins Struct. Funct. Genet. 11, 281±296 (1991). 4. Allsop, A. E. et al.inAnti-Infectives, Recent Advances in Chemistry and Structure-Activity Relationships 30. Meritt, E. A. & Bacon, D. J. Raster3D: photorealistic molecular graphics. Methods Enzymol. 277, 505± (eds Bently, P. H. & O'Hanlon, P. J.) 61±72 (R. Soc. Chem., Cambridge, 1997). -

Xuejun Yu Dissertation Final
UNIVERSITY OF CALIFORNIA RIVERSIDE Conversion of Carbon Dioxide to Formate by a Formate Dehydrogenase from Cupriavidus necator A Dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Bioengineering by Xuejun Yu September 2018 Dissertation Committee: Dr. Ashok Mulchandani, Co-Chairperson Dr. Xin Ge, Co-Chairperson Dr. Russ Hille Copyright by Xuejun Yu 2018 The Dissertation of Xuejun Yu is approved: Committee Co-Chairperson Committee Co-Chairperson University of California, Riverside ACKNOWLEDGEMENTS I would like to express sincere appreciation to my advisor, Professor Ashok Mulchandani, for accepting me to be his student when I was suffering. Thank you very much for your delicate guidance, support and encouragement during the past years. You not only guide me on my PhD study, but also help me to be mature on my personality. From bottom of my heart, I feel very lucky to have you as professor. Also, many thanks go to my other committee members. Professor Xin Ge acted as my co-advisor and provided many valuable comments on my molecular cloning work. Professor Russ Hille has also provided insights, encouragements and advices as a member of my committee members. I have learned so many important insights from our meetings and discussions and thank you for bringing me into the molybdenum/tungsten enzyme conference. I am also thankful to the past and current group members, colleagues and friends - Dimitri Niks, Pankaj Ramnani, Feng Tan, Rabeay Hassan, Trupti Terse, Thien-Toan Tran, Claudia Chaves, Jia-wei Tay, Hui Wang, Pham Tung, Hilda Chan, Yingning Gao and Tynan Young.