3 ANIMAL BONES: DATA by Andy Hammon 3.1 Appendix 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Kelly Mantle
The VARIETY SHOW With Your Host KELLY MANTLE KELLY MANTLE can be seen in the feature film Confessions of a Womanizer, for which they made Oscars history by being the first person ever to be approved and considered by The Academy for both Supporting Actor and Supporting Actress. This makes Kelly the first openly non-binary person to be considered for an Oscar. They are also featured in the movie Middle Man and just wrapped production on the upcoming feature film, God Save The Queens in which Kelly is the lead in. TV: Guest-starred on numerous shows, including Lucifer, Modern Family, Curb Your Enthusiasm, CSI, The New Normal, New Adventures of Old Christine, Judging Amy, Nip/Tuck, Will & Grace, George Lopez. Recurring: NYPD Blue. Featured in LOGO’s comedy special DragTastic NYC, and a very small co-star role on Season Six of RuPaul's Drag Race. Stage: Kelly has starred in more than 50 plays. They wrote and starred in their critically acclaimed solo show,The Confusion of My Illusion, at the Los Angeles LGBT Center. As a singer, songwriter, and musician, Kelly has released four critically acclaimed albums and is currently working on their fourth. Kelly grew up in Oklahoma like their uncle, the late great Mickey Mantle. (Yep...Kelly's a switch-hitter, too.) Kelly received a B.F.A. in Theatre from the University of Oklahoma and is a graduate of Second City in Chicago. https://www.instagram.com/kellymantle • https://www.imdb.com/name/nm0544141/ ALEXANDRA BILLINGS is an actress, teacher, singer, and activist. -

Curiosity Guide #306 Skeletal System
Curiosity Guide #306 Skeletal System Accompanies Curious Crew, Season 3, Episode 6 (#306) Calcium-Rich Bones Investigation #6 Description Explore the role of calcium in making strong bones. Materials 6 chicken bones 6 containers Calcium tablets Vinegar Procedure 1: Prepare the bones 1) Boil the bones to remove any meat. 2) Soak the bones in 1 part bleach to ten parts water for five minutes. 3) Let the bones dry. Procedure 2: Investigate 1) Place one chicken bone in each of the 6 glasses. 2) Pour vinegar over each bone. 3) Do not add any calcium to the first container. 4) In the second container, add 300 milligrams of calcium. 5) In the third container, add 600 mg calcium, 1,200 mg in the fourth, 1,800 mg in the fifth, and 2,400 mg in the sixth. 6) Leave the bones for 5 days. 7) Compare the results. 8) Drop a calcium tablet into a cup of vinegar. How does it react? My Results Explanation In addition to calcium, bones also contain phosphorous. However, it is the calcium salts that make bones rigid. Acids such as vinegar can dissolve those calcium salts and leave the bone soft and rubbery. In this experiment, the more calcium that was in the container, the stronger the bones remained. Because the mineral calcium provides rigidity to the bones, it is important to eat calcium rich foods like low fat dairy; green, leafy vegetables like collard greens; beans; and nuts. A person between the ages of 11 and 24 should consume 1,200 milligrams of calcium every day. -
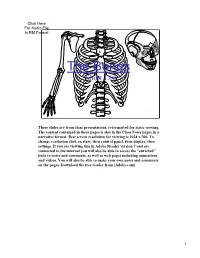
The Bones Short List
The Bones Short List 1 These slides are from class presentations, reformatted for static viewing. The content contained in these pages is also in the Class Notes pages in a narrative format. Best screen resolution for viewing is 1024 x 768. To change resolution click on start, then control panel, then display, then settings. If you are viewing this in Adobe Reader version 7 and are connected to the internet you will also be able to access the “enriched” links to notes and comments, as well as web pages including animations and videos. You will also be able to make your own notes and comments on the pages. Download the free reader from [Adobe.com] 1 Types of Bones 1) Long bones – bones of the upper and lower arms and legs as well as the metacarpals and metatarsals. 2) flat bones – sternum, ribs, scapulae, cranium 3) short bones – carpals and tarsals 4) irregular bones – vertebrae 5) sesamoid bones – patellae 2 2 Skeletal Divisions 1) Axial skeleton – skull, sternum, ribs, and vertebrae (including sacrum) 2) Appendicular skeleton – bones of the pectoral and pelvic girdles and of the arms, legs, wrists, hands, ankles, and feet. 3 3 Figure 7.3 a Frontal (coronal) suture Sphenoid Parietal bone bone Squamosal Frontal bone (temporal) suture Occipital (lamdoidal) Nasal bone suture Zygomatic bone Occipital bone Maxilla Temporal bone Mandible 4 Important: the mastoid process (important site for attachment of muscles such as the sternocleidomastoid), the external acoustic meatus (opening into the parts of the ear), the articulation of the temporal bone and mandible ( the TMJ), and the zygomatic arch, all on the temporal bone. -

Download Bare Bones, Kathy Reichs, Random House, 1999
Bare Bones, Kathy Reichs, Random House, 1999, , . DOWNLOAD http://archbd.net/17PVC8q Bones are Forever , Kathy Reichs, 2013, Fiction, 414 pages. The gripping new Temperance Brennan novel from the world class forensic anthropologist and Number 1 bestselling author.A newborn baby is found wedged in a vanity cabinet in a .... The Law of Betrayal , Tess Collins, Jun 30, 2006, Fiction, 292 pages. When she was ten years old Alma's father disappeared. The only man who knows the true story is brutally killed, but she must defend herself against an accusation of murder.. Break No Bones A Novel, Kathy Reichs, Jul 11, 2006, Fiction, 352 pages. Following the tremendous success of Cross Bones, Kathy Reichs explores another high-profile topic in Break No Bones -- a case that lands forensic anthropologist Temperance .... Bones Buried Deep, Max Allan Collins, Kathy Reichs, Feb 28, 2006, Fiction, 304 pages. Forensic anthropologist Dr. Termperance Brennan is called in by Special Agent Seeley Booth to assist in the investigation into a bag of skeletal remains, complete with note .... Cross Bones , Kathy Reichs, May 23, 2006, Fiction, 496 pages. Receiving mysterious clues about a shooting murder in Montreal, Tempe Brennan wonders if the victim may have been a Jewish black market antiquities trader and teams up with .... Deadly Decisions A Novel, Kathy Reichs, Aug 8, 2000, Fiction, 336 pages. Nobody tells a chilling story like international bestselling author Kathy Reichs, whose "most valuable tool is her expertise...she's the real thing" (New York Newsday). Drawing .... Corpi freddi La serie di Temperance Brennan #1, Kathy Reichs, , Fiction, 362 pages. -

Bugs, Bones & Botany Workshop October 30-November 4, 2016 Gainesville, Florida
Bugs, Bones & Botany Workshop October 30‐November 4, 2016 Gainesville, Florida October 30, 2016 Classroom Lecture Topics 1. Entomology: History & Overview of Entomology, Dr. Jason 8AM‐12 PM Byrd 2. Anthropology: Introduction to Forensic Anthropology, Instructor: Dr. Mike Warren 3. Botany: Using Botanical Evidence in a Forensic Investigation, Instructor: Dr. David Hall 4. Crime Scene: Search & Field Recovery Techniques, Gravesite excavation, begin 1‐5 PM Field Topic 1. Entomology: Field Demonstration of Collection Procedures, Instructor: Dr. Jason Byrd 2. Anthropology: Hands‐on Skeletal Analysis Instructor: Dr. Mike Warren 3. Botany: Where Plants Grow & Mapping/Collecting Equipment, Instructor: Dr. David Hall November 1, 2016 Classroom Topic 1. Entomology: Processing a Death Scene for Entomological 8‐12 AM Evidence, Instructor: Dr. Jason Byrd 2. Anthropology: Human and Nonhuman Skeletal Anatomy, Instructor: Dr. Mike Warren 3. Botany: Characteristics of Plants, Instructor: Dr. David Hall 1‐5 PM Field topic 1. Entomology: Collection of Entomological Evidence Instructor: Dr. Jason Byrd 2. Anthropology: Students will excavate buried remains, Instructor: Dr. Mike Warren 3. Botany: Collecting Botanical Evidence & Mapping Surface Scatter, Instructor: Dr. David Hall November 2, 2016 Classroom Topic 1. Entomology: Estimation of PMI Using Entomological Evidence, 8 AM – 12 PM Instructor: Dr. Jason Byrd 2. Anthropology: Methods of Human Identification Instructor: Dr. Mike Warren 3. Botany: When to call a Forensic Botanist Instructor: Dr. David Hall 1‐5 PM Field Topic 1. Entomology: Continue Processing of Entomological Evidence, Instructor: Dr. Jason Byrd 2. Anthropology: Continue Processing Anthropological Evidence, Instructor: Dr. Mike Warren 3. Botany: Continue Processing Botanical Evidence & Surface Scatter, Instructor: Dr. David Hall November 3, 2016 Classroom Topic 1. -

How I Set up My Own Body Farm by Jennifer Dean
How I Set Up My Own Body Farm By Jennifer Dean To prepare for a new forensic science elective at Camas High School, and determined to make this new course an exciting application of biology and chemistry principles, I began by collecting forensic science resources, ordering books and enrolling in the local community college course on forensic science. I spent every extra hour soaking up as much as I could about this field during the “time off” teachers get in the summer. I was particularly fascinated by the fields of forensic entomology and anthropology. From books such as Stiff: The Curious Lives of Human Cadavers by Mary Roach and Dr. Bill Bass’s work around the creation of a human body farm at the University of Tennessee Forensic Anthropology Center, I decided to create something similar for our new high school forensic science program. As we met for dinner after a day of revitalizing workshops in Seattle at an NSTA conference, I shared my thoughts about the creation of an animal body farm with my talented and dedicated colleagues. These teachers have a passion for their students and their work. I felt free to share these ideas with them and know I’d be supported in making it a reality. As the Science Department, we formally agreed to dedicate ourselves to submitting grants to make it a reality. Back at work, I sent copies of the farm proposal to my immediate supervisors, and they responded with letters of support. The next step was getting the farm started—with or without grant money—because the first class of forensic science would be starting in the fall. -

1446674611085.Pdf
1 Play-Testers In no particular order: Alex Henzler, Bill Logan, Hunter Logan, Javan M. Kelly, Justin Mills, Kelly Reed, Mikey Credits Kane, Nancy Ludden, Noah Logan, Rich Peterson, Robert Design Larry Moore Ludden, Sean Michael Kelly, Tammy Moore, Tiras H.M. Co-Design Bill Logan Kelly, Vince Kane, Vinnie Kane, Big Al, Rob Ludden, Nancy Editing Jim White Ludden, Jim Pelton, Kyrinn S. Eis, Tre’ Grisby, Mark Tammy Moore Hassman, Gaston Gosselin, Jeremy Whalen, Jayce Gaines, Gaston R. Gosselin Michael Hogaboom, R.E. Davis Cover Art Eric Quigley Illustrations William Stewart About the Author Khairul Hisham Larry Moore is a network/security engineer by day, a Bill Logan calling given to him without repentance. By night he Larry Moore morphs into a game crafter, fanzine editor or adventure Jeremy Whalen writer. You might know him from a Con in the Ohio area, the Star Frontiersman fanzine or the design of the up- coming Frontier Space RPG. You might know him for other reasons that are best left for history to write. If you don’t know Larry, he would love to hear from you. Email him at [email protected] or Twitter @simplymenotu or G+ http://gplus.to/w00t About DwD Studios After several years of digitally re-mastering our favorite science-fiction game and publishing years of issues of the free online fanzine, the Star Frontiersman, Bill Logan and Larry Moore decided it was time to put their creative efforts together and make games, lots of games! But not just any games, we have blended the best of the "old school" games with the best of the "new school" games to create fresh ideas and unique products. -

Sea-Monkey Necklace” Rades, and Other Debris
The B l o t t e r “Almanac Entry” G. M. Somers ....................Editor-in-Chief Martin K. Smith...........Publisher-at-Large, It’s 64 degrees Fahrenheit in our living room – because I’m stubborn, Treasurer mostly, and also because no one else is home at the moment and I don’t Marilyn Fontenot......................Director of need it to be particularly warm to type here. (It’s cooler outside, but still Development November-ish sunny and I’m considering changing venues for writing this Laine Cunningham...................Publishing Consultant document.) I have a cup of tea - cream and sugar, thanks - and it’s help- Brace Boone III............Marketing Advisor ing me maintain appropriate body warmth. I have a sweatshirt on, also, Richard Hess.................Programs Director but the sleeves are rolled up, figuratively and literally. Today has been a T.J. Garrett....................Staff Photographer very good day. It started off iffy enough. (I like that last sentence a lot. Say it out Subscriptions Contact: loud, three times, fast.) For reasons I cannot easily comprehend, although Martin K. Smith “tired” leaps to mind, I went to bed at 7:30 last night. Fell almost imme- [email protected] 919.286.7760 diately asleep, woke up ridiculously early this morning and got to work. Advertisers Contact: I’d thought I would read – I mean really read, for a few hours. Serious, Martin K. Smith uncompromising consumption of text, like so many folks I know say (on [email protected] Twitter of all things) that they are able to do. “I blasted through that 919.286.7760 book this weekend. -

December 2015 Client Alert: Bones Complaints: Did Fox Properly Account for Hulu Monies? by Ezra Doner* Twentieth Century Fox
December 2015 Client Alert: Bones Complaints: Did Fox Properly Account for Hulu monies? By Ezra Doner* Twentieth Century Fox produces the hit TV series Bones for its broadcast network and also makes the series available on Hulu of which it is a one-third owner. Did Fox properly report and share monies it received from its license of Bones to Hulu? Two complaints recently filed by revenue participants claim it did not.1 The Big Picture As new media technologies and services continue to develop, revenue participants want the opportunity to shape corresponding licensing practices. This is especially true when a distributor, such as Fox, licenses to a new media company, such as Hulu, which Fox co-owns. These new Bones cases raise issues I previously explored in posts about The Walking Dead and Harlequin Books. Bones, the Series Bones, a one-hour crime procedural, debuted on the Fox broadcast network in September 2005 and is now in its 11thseason, making it Fox’s longest running one-hour drama ever. As of the date of this writing, a remarkable 220 episodes have aired. The TV series is based on the “Temperance Brennan” book franchise by Kathy Reichs, a forensic anthropologist. The stars of the series are Emily Deschanel as Brennan and David Boreanaz as Agent Booth. Barry Josephson, an originator of the series, has been an executive producer since inception. The Plaintiffs Reichs, Deschanel and Boreanaz are plaintiffs in one of the recent complaints, and Josephson is the plaintiff in the other. Per the complaints, the plaintiffs’ profits participations are: Reichs, 5%; Deschanel and Boreanaz, 3% each; and Josephson, a “significant” but unspecified percentage. -

The Bare Bones of Social Commentary in Kathy Reichs’ Fiction
THE BARE BONES OF SOCIAL COMMENTARY IN KATHY REICHS’ FICTION Carme Farre-Vidal Universidad de Lérida Abstract Resumen Detective fiction has popularly been Tradicionalmente, la novela policíaca ha sido considered a light form of literary considerada como una forma literaria de entertainment. However, many of this mero entretenimiento e intranscendente. Sin genre’s practitioners underline the embargo, muchos de los escritores de este way that their novels engage with género subrayan que sus novelas están contemporary social issues, as a close comprometidas con las cuestiones sociales reading of the texts may reveal. Kathy contemporáneas, tal y como se desprende de Reichs’ fiction is no exception. In this una lectura atenta de sus textos. En este sense, her Brennan series may be sentido, la novelística de Kathy Reichs no es analysed as prompting the reader to una excepción y su serie Brennan puede set out on a journey of discovery in plantearse como una forma de ficción que different ways. This article argues that busca trascender y suscitar en el lector un content and form work hand in hand viaje iniciático. Este artículo sostiene que at the service of Kathy Reichs’ social contenido y forma tienden a equiparar la feminist agenda and that just as the actividad forense y la agenda feminista de many times bare bones found at the Kathy Reichs, y que, así como en la primera crime scene point to both the victim’s los huesos humanos encontrados en la escena and criminal’s identity, they del crimen revelan tanto la identidad de la eventually become suggestive of how víctima como la del criminal, la segunda our contemporary society works. -

Health Effects of Drug Abuse
Health Risks of Drug Abuse Medical Consequences of Drug Abuse From the National Institute on Drug Abuse : Drug addiction is a brain disease. Although initial drug use might be voluntary, drugs of abuse have been shown to alter gene expression and brain circuitry, which in turn affect human behavior. Once addiction develops, these brain changes interfere with an individual’s ability to make voluntary decisions, leading to compulsive drug craving, seeking and use. The impact of addiction can be far reaching. Cardiovascular disease, stroke, cancer, HIV/AIDS, hepatitis, and lung disease can all be affected by drug abuse. Some of these effects occur when drugs are used at high doses or after prolonged use; however, some may occur after just one use. HIV, Hepatitis and Other Infectious Diseases Drug abuse not only weakens the immune system but is also linked to risky behaviors like needle sharing and unsafe sex. The combination greatly increases the likelihood of acquiring HIV-AIDS, hepatitis and many other infectious diseases. Drugs that can lead to HIV, Hepatitis and other infectious diseases: Heroin Cocaine Steroids Methamphetamine Cardiovascular Effects Researchers have found a connection between the abuse of most drugs and adverse cardiovascular effects, ranging from abnormal heart rate to heart attacks. Injection drug use can also lead to cardiovascular problems such as collapsed veins and bacterial infections of the blood vessels and heart valves. Drugs that can affect the cardiovascular system: Cocaine Heroin Inhalants Ketamine LSD Marijuana MDMA Methamphetamine Nicotine PCP Prescription Stimulants Steroids Respiratory Effects Drug abuse can lead to a variety of respiratory problems. -

The Nikumaroro Bones Identification Controversy: First-Hand Examination Versus Evaluation by Proxy — Amelia Earhart Found Or Still Missing?
The Nikumaroro bones identification controversy: First-hand examination versus evaluation by proxy — Amelia Earhart found or still missing? Item Type Article Authors Cross, Pamela J.; Wright, R. Citation Cross PJ and Wright R (2015) The Nikumaroro bones identification controversy: First-hand examination versus evaluation by proxy — Amelia Earhart found or still missing? Journal of Archaeological Science: Reports. 3: 52-59. Rights (c) 2015 Elsevier Ltd. All rights reserved. Full-text reproduced in accordance with the publisher's self-archiving policy. Download date 01/10/2021 02:19:54 Link to Item http://hdl.handle.net/10454/7286 The Nikumaroro Bones Identification Controversy: First-hand Examination versus Evaluation by Proxy – Amelia Earhart Found or Still Missing? Pamela J. Cross1* and Richard Wright2 *1Archaeological Sciences, University of Bradford, Bradford UK, [email protected] 2Emeritus Professor of Anthropology, University of Sydney, Australia Abstract American celebrity aviator Amelia Earhart was lost over the Pacific Ocean during her press-making 1937 round-the-world flight. The iconic woman pilot remains a media interest nearly 80 years after her disappearance, with perennial claims of finds pinpointing her location. Though no sign of the celebrity pilot or her plane have been definitively identified, possible skeletal remains have been attributed to Earhart. The partial skeleton recovered and investigated by British officials in 1940. Their investigation concluded the remains were those of a stocky, middle-aged male. A private historic group re- evaluated the British analysis in 1998 as part of research to establish Gardner (Nikumaroro) Island as the crash site. The 1998 report discredited the British conclusions and used cranial analysis software (FORDISC) results to suggest the skeleton was potentially a Northern European woman, and consistent with Amelia Earhart.