Hypertension in Pregnancy Hypertension
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Association of Induced Abortion with Hypertensive Disorders Of
www.nature.com/scientificreports OPEN Association of induced abortion with hypertensive disorders of pregnancy risk among nulliparous women in China: a prospective cohort study Yinhua Su1,2, Xiaoping Xie3, Yanfang Zhou1, Hong Lin1, Yamei Li1, Na Feng1 & Jiayou Luo1* The relationship between induced abortion(IA) and hypertensive disorders of pregnancy(HDP) is inconclusive. Few studies have been conducted in China. In order to clarify the association between previous IA and risk of HDP, including gestational hypertension(GH) and pre-eclampsia(PE), we performed a community-based prospective cohort study enrolling 5191 eligible nulliparous women in selected 2 districts and 11 towns of Liuyang from 2013 to 2015. Multivariable logistic regression was conducted to examine whether IA was associated with HDP, GH and PE. Of the gravidea, 1378(26.5%) had a previous IA and 258(5.0%) diagnosed with HDP, including 141(2.7%) GH and 117(2.3%) PE. The diference in the incidence of GH and PE between gravidae having one versus those with two or more IAs was minimal. After adjustment for maternal age, body mass index at frst antenatal visit, education, virus infection and history of medical disorders, previous IA was signifcantly associated with HDP (OR = 0.67, 95%CI = 0.49 to 0.91) and PE (OR = 0.61, 95%CI = 0.38 to 0.97), but not with GH (OR = 0.73, 95%CI = 0.49 to 1.10). Additional adjustment for occupation, living area, anemia, gestational diabetes mellitus, psychological stress, conception climate and infant sex, multivariable analysis provided similar results. In conclusion, previous IA was associated with a lower risk of PE among nulliparous women. -
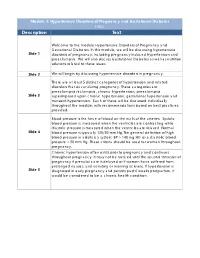
Module 2: Hypertensive Disorders of Pregnancy and Gestational Diabetes FINAL Description Text
Module 2: Hypertensive Disorders of Pregnancy and Gestational Diabetes FINAL Description Text Welcome to the module Hypertensive Disorders of Pregnancy and Gestational Diabetes. In this module, we will be discussing hypertensive Slide 1 disorders of pregnancy, including pregnancy induced hypertension and preeclampsia. We will also discuss Gestational Diabetes as well as nutrition solutions related to these issues Slide 2 We will begin by discussing hypertensive disorders in pregnancy. There are at least 5 distinct categories of hypertension and related disorders that occur during pregnancy. These categories are: preeclampsia/eclampsia, chronic hypertension, preeclampsia Slide 3 superimposed upon chronic hypertension, gestational hypertension and transient hypertension. Each of these will be discussed individually throughout the module, with recommendations based on best practices provided. Blood pressure is the force of blood on the walls of the arteries. Systolic blood pressure is measured when the ventricles are contracting while diastolic pressure is measured when the ventricles are relaxed. Normal Slide 4 blood pressure is typically 120/80 mm Hg.The general definition of high blood pressure in adults is a systolic BP > 140 mg HG or a diastolic blood pressure > 90 mm Hg. These criteria should be used for women throughout pregnancy. Chronic hypertension often exists prior to pregnancy and continues throughout pregnancy. It may not be noticed until the second trimester of pregnancy if prenatal care is delayed or if women have suffered from prolonged nausea and vomiting or morning sickness. If hypertension is Slide 5 diagnosed in early pregnancy and persists past 6 weeks postpartum, it would be considered to be a chronic health condition. -

Placental Abruption in Each Phenotype of Hypertensive Disorders of Pregnancy: a Retrospective Cohort Study Using a National Inpatient Database in Japan
Hypertension Research (2021) 44:250–252 https://doi.org/10.1038/s41440-020-00557-2 COMMENT Placental abruption in each phenotype of hypertensive disorders of pregnancy: a retrospective cohort study using a national inpatient database in Japan 1 1,2 2,3 Nelson Sass ● Gilberto Nagahama ● Henri Augusto Korkes Received: 24 August 2020 / Revised: 2 September 2020 / Accepted: 3 September 2020 / Published online: 7 October 2020 © The Japanese Society of Hypertension 2020 In a very interesting paper, Naruse et al. [1] analyzed a ret- Therefore, it is plausible to claim that PE is also a “bloody rospective cohort from a Japanese database, identifying a business” (Fig. 1). higher incidence of placental abruption in women with severe Advances in the knowledge of the pathological changes preeclampsia (PE) when admitted at less than 34 weeks associated with PE, especially in the early onset forms, gestational age (GA). Abruption of the placenta is an obstetric suggest that poor trophoblastic invasion in the early stages emergency that is usually catastrophic and requires prompt triggers an inflammatory syndrome with abnormal meta- 1234567890();,: 1234567890();,: diagnosis and immediate intervention, usually by emergency bolic pathways, including placental release of large amounts cesarean section, to save the fetus and reduce the risk of antiangiogenic factors such as soluble forms-like tyrosine of bleeding complications in the mother. The results from kinase-1 (sFlt-1). High plasma levels of these factors hinder Naruse et al. [1] highlight that this serious condition has the action of vascular endothelial growth factor (VEGF) and occurred after hospital admission in expectant patients with placental growth factor (PLGF) on receptors on the endo- early onset PE at a GA less than 34 weeks. -

Pregnancy Problems in Primary Care
Moneli Golara Consultant Obstetrician and Gynaecologist Barnet Hospital 680000 babies born in the UK Pregnancy associated with change of physiology Changes results in common symptoms of pregnancy for which patients may see health care professionals Considered a ‘stress test’ on womens’ health Organ specific Skin Mucous membranes Cardiovascular Respiratory Renal GI Endocrine Specific conditions in pregnancy with cases Pigmentation Pathogenesis not completely understood Linea alba becomes nigra Areola darkens Axilla, genitalia, perineum, anus, inner thighs and neck Recent scars, freckles May take several months to recover postpartum Naevi can look suspicious during pregnancy Melasma occurs in 75% of women Oestrogen causes vascular distension and proliferation of blood vessels Spider angiomas and Naevi Mostly resolve within 3 months Palmar erythema Varicosities Venous distension of vestibule and Vagina and vulval varicosities Oedema and water retention of extremeties Purpura Striae gravidarum Hirsuitism Arms, legs back and suprapubic region Anagen and telogen hair Scalp hair appears thicker during pregnancy but hair loss and thinning 1-5 months post partum This may resolve by 15 months but never to the same state in some people Androgen alopecia involving frontal scalp may occur Blue discolouration of vagina and cervix Gingival changes, and gingivitis, bleeding ulceration and pain Hyperaemia of nasal mucosa causing congestion CO Plasma volume To encourage optimum growth of fetus Increase in red cell -

Gestational Hypertension & Pre-Eclampsia
Journal of Labor and Childbirth Gestational hypertension & pre-eclampsia Introduction • First, we talk about the definitions • When, how, why the disease occurs? • How diagnose, how to treat and how to prevent according to guidelines Moatasem Bellah Al Farrah • This lecture is to know about the disease in new way. DUMMAR Medical Center, Syria Background : Prompt identification and appropriate management of Hypertensive Disorders in Pregnancy (HDP) are essential for optimal outcomes because HDP: Biography • Are associated with severe maternal obstetric complications and increased maternal mortality risk Moatasem Bellah Al Farrah is a mem- ber of Syrian Doctors Association. He is • Lead to preterm delivery, fetal intrauterine growth restriction, low birthweight and perinatal death currently working as an assistant profes- sor in Dummar Medical center and Red Study made between 2006 and 2008 showed: 70 maternal deaths, showing leading causes of death to Crescent. He is also a member in British be: hypertension (20%), haemorrhage (19%) and embolism (17%). Chronic illness, obesity and prenatal Society for Gynecological Endoscopy, International Urogynecology Associa- risk factors were identified as important circumstances in the cases reviewed. tion, British Society for Urogynecolo- Definition and classification of Hypertensive Disorders in Pregnancy (HDP) gy. His professional interests focus on PCOS and Endometriosis Researches. Hypertensive Disorders in Pregnancy are comprised of a spectrum of disorders typically classified into categories -

Original Articles
Bangladesh Journal of Anatomy January 2014, Vol. 12, No. 1 pp. 3-6 Original Articles Study of Umbilical Cord in Pregnancy Induced Hypertension with and without Diabetes Mellitus Rita Rani Saha1, Nazma Farhat2, Mallika Karmaker3 Abstract Context : Two organs that can provide a good insight into pregnancy induced hypertension with and without diabetes mellitus are the placenta and the umbilical cord along with their vessels. In the present study the umbilical cord is taken from the placenta of hypertensive and diabetic mothers to measure its diameter, to observe contained vessels and its mode of attachment to the placenta. Materials and Methods: An observational and analytical type of study was conducted in the department of Anatomy, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka between November 2003 to May 2004 on umbilical cords. To study the umbilical cord, placentas were collected from fifty eight Bangladeshi women who gave birth to a single live baby through Caesarian section after 35 to 40 weeks of gestasion. Twenty of the mothers were non hypertensive, non diabetic control, twenty had pregnancy induced hypertension(PIH) and eighteen had pregnancy induced hypertension and gestesional diabetes mellitus(PIH+GDM). Results: No significant difference was found between any groups for diameter of the umbilical cord. All the umbilical cords had same number of vessels. There was variation among the mode of insertion of umbilical cord. Conclusion: Diameter of umbilical cords did not show significant difference in either of the two diseased groups with the control group in the Post Hoc option of analysis of variance (ANOVA) at 5% level. Key words: Umbilical cord, pregnancy induced hypertension, gestational diabetes mellitus. -

Guideline for the Management of Hypertensive Disorders of Pregnancy 2014
Guideline for the Management of Hypertensive Disorders of Pregnancy 2014 Lowe SA, Bowyer L, Lust K, McMahon LP, Morton M, North RA, Paech M. Said JM. These are the recommendations of a multidisciplinary working party convened by the Society of Obstetric Medicine of Australia and New Zealand. They reflect current medical literature and the clinical experience of members of the working party. CONTENTS Section Page Abbreviations 2 1. Definition of hypertension in pregnancy 3 2. Recording blood pressure in pregnancy 4 3. Classification of hypertensive disorders in pregnancy 5 4. Investigation of new onset hypertension after 20 weeks 9 5. Management of preeclampsia and gestational hypertension 11 6. Eclampsia 18 7. Fetal Surveillance in hypertensive diseases of pregnancy 19 8. Resolution of preeclampsia and gestational hypertension 21 9. Chronic hypertension in pregnancy 22 10. Anaesthetic considerations 26 11. Preconception management and prophylaxis 28 12. Prevention of preeclampsia 30 13. Longterm consequences 33 14. Auditing outcomes 34 15. References 36 1 ABBREVIATIONS ABPM Ambulatory blood pressure monitoring AFV Amniotic fluid volume ALT Alanine transaminase AOR Adjusted odds ratio APPT Activated partial thromboplastin time AST Aspartate transaminase BW Birth weight CI Confidence Interval ECG Electrocardiogram FBC Full blood count FGR Fetal growth restriction HELLP Haemolysis, elevated liver enzymes and low platelet syndrome Hr Hour(s) INR International normalised ratio ISSHP International Society for the Study of Hypertension in Pregnancy -

Gestational Hypertension and Preeclampsia Hypertensive Disorders of Pregnancy Constitute One of the Leading Causes of Maternal and Perinatal Mortality Worldwide
INTERIM UPDATE ACOG PRACTICE BULLETIN Clinical Management Guidelines for Obstetrician–Gynecologists NUMBER 222 (Replaces Practice Bulletin No. 202, December 2018) Committee on Practice Bulletins—Obstetrics. This Practice Bulletin was developed by the American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics in collaboration with Jimmy Espinoza, MD, MSc; Alex Vidaeff, MD, MPH; Christian M. Pettker, MD; and Hyagriv Simhan, MD. INTERIM UPDATE: The content of this Practice Bulletin has been updated as highlighted (or removed as necessary) to include limited, focused editorial corrections to platelet counts, diagnostic criteria for preeclampsia (Box 2), and pre- eclampsia with severe features (Box 3). Gestational Hypertension and Preeclampsia Hypertensive disorders of pregnancy constitute one of the leading causes of maternal and perinatal mortality worldwide. It has been estimated that preeclampsia complicates 2–8% of pregnancies globally (1). In Latin America and the Caribbean, hypertensive disorders are responsible for almost 26% of maternal deaths, whereas in Africa and Asia they contribute to 9% of deaths. Although maternal mortality is much lower in high-income countries than in developing countries, 16% of maternal deaths can be attributed to hypertensive disorders (1, 2). In the United States, the rate of preeclampsia increased by 25% between 1987 and 2004 (3). Moreover, in comparison with women giving birth in 1980, those giving birth in 2003 were at 6.7-fold increased risk of severe preeclampsia (4). This complication is costly: one study reported that in 2012 in the United States, the estimated cost of preeclampsia within the first 12 months of delivery was $2.18 billion ($1.03 billion for women and $1.15 billion for infants), which was disproportionately borne by premature births (5). -

Raised Blood Pressure in Pregnancy: Gestational Hypertension
Maternity information Raised blood pressure in pregnancy: Gestational hypertension This leaflet is for women who have high blood pressure brought on by their pregnancy, usually appearing after week 20 of their pregnancy – known as ‘gestational hypertension’. The leaflet explains what it is, how it is monitored and how it is treated during pregnancy. If you have any questions or concerns, please speak to your midwife or doctor. What is gestational hypertension? If a woman develops hypertension in pregnancy for the first time (usually after 20 weeks) this is called gestational hypertension. Gestational hypertension may be either transient hypertension of pregnancy (a temporary condition) or chronic hypertension (undiagnosed pre-existing condition) that is identified first time in the latter half of pregnancy. What is hypertension in pregnancy? Normal pregnancy is associated with a natural fall in blood pressure. High blood pressure or hypertension occurs in 10-15% of all pregnancies. It is defined as a blood pressure of 140 mm Hg (the top number) or 90 mm Hg (the bottom number) (140 over 90) in women known to have normal blood pressure before pregnancy. Most people cannot feel their blood pressure although sometimes hypertension will cause symptoms. Please notify your midwife, obstetrician, GP urgently if you develop headache, blurred vision and upper abdominal pain. Why does hypertension matter? Developing hypertension in pregnancy (or shortly after birth) is associated with increased complications for both mother and baby. It is therefore important to monitor your pregnancy more frequently if you develop hypertension in pregnancy. When hypertension causes other effects such as abnormal kidney or liver function or reduced weight gain for your baby, it is called pre-eclampsia. -

Hypertensive Disorders of Pregnancy
Hypertensive Disorders of Pregnancy LAWRENCE LEEMAN, MD, MPH, University of New Mexico School of Medicine, Albuquerque, New Mexico PATRICIA FONTAINE, MD, MS, University of Minnesota Medical School, Minneapolis, Minnesota The National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy has defined four categories of hypertension in pregnancy: chronic hypertension, gestational hypertension, preeclampsia, and preeclampsia superimposed on chronic hypertension. A maternal blood pressure measurement of 140/90 mm Hg or greater on two occasions before 20 weeks of gestation indicates chronic hypertension. Pharmacologic treatment is needed to prevent maternal end-organ damage from severely elevated blood pressure (150 to 180/100 to 110 mm Hg); treatment of mild to moderate chronic hypertension does not improve neonatal outcomes or prevent superimposed preeclampsia. Gestational hypertension is a provisional diagnosis for women with new-onset, nonproteinuric hyper- tension after 20 weeks of gestation; many of these women are eventually diagnosed with preeclampsia or chronic hypertension. Preeclampsia is the development of new-onset hypertension with proteinuria after 20 weeks of gesta- tion. Adverse pregnancy outcomes related to severe preeclampsia are caused primarily by the need for preterm deliv- ery. HELLP (i.e., hemolysis, elevated liver enzymes, and low platelet count) syndrome is a form of severe preeclampsia with high rates of neonatal and maternal morbidity. Magnesium sulfate is the drug of choice to prevent and treat eclampsia. The use of magnesium sulfate for seizure prophylaxis in women with mild preeclampsia is controversial because of the low incidence of seizures in this population. (Am Fam Physician. 2008;78(1):93-100. -

Pregnancy: Occupational Aspects of Management: Concise Guidance
■ CONCISE GUIDANCE Clinical Medicine 2013, Vol 13, No 1: 75–9 Pregnancy: occupational aspects of management: concise guidance Keith T Palmer, Matteo Bonzini and Jens-Peter Ellekilde Bonde, on behalf of a multidisciplinary Guideline Development Group convened by, and in association with, the Health and Work Development Unit, a collaboration between the Royal College of Physicians and the Faculty of Occupational Medicine ABSTRACT – Most pregnant women are exposed to some work are well established (eg from ionising radiation and lead) physical activity at work. This Concise Guidance is aimed at and particular strategies have been developed to manage the doctors advising healthy women with uncomplicated singleton associated risks. However, there are other potential workplace pregnancies about the risks arising from five common work- hazards for which the scientific evidence is less certain. place exposures (prolonged working hours, shift work, lifting, Important among these is the possibility that physical activi- standing and heavy physical workload). The adverse outcomes ties at work might adversely affect outcomes of pregnancy.3 It considered are: miscarriage, preterm delivery, small for gesta- has been suggested, for example, that women’s work schedules tional age, low birth weight, pre-eclampsia and gestational (including rotating shifts and night work) can induce neuroen- hypertension. Systematic review of the literature indicates docrine changes as a consequence of sleep deprivation or dis- that these exposures are unlikely to carry much of an increased rupted circadian rhythms, affecting fetal growth and the timing risk for any of the outcomes, since small apparent effects of parturition. In theory, long working hours, prolonged might be explicable in terms of chance, bias, or confounding, standing, heavy lifting or an unusual workload might also pose while larger and better studies yield lower estimated risks several threats to pregnant workers. -

Hypertension in Pregnancy Care Process Model
Minnesota Perinatal Quality Collaborative Hypertension in Pregnancy Care Process Model HYPERTENSION IN PREGNANCY CARE PROCESS MODEL Resources for Providers, Nurses, and Health Care Consumers Disclaimer: Applicability of all information within is subject to change based on current literature and was last accurate as of 7.24.2020. Document will be reviewed every six months. Page | 1 MNPQC Subcommittee for Maternal Hypertension Approved for use 7.24.20 MinnesotaMinnesota Perinatal Perinatal Quality Quality Collaborative Collaborative HypertensionHypertension in Pregnancyin Pregnancy Care Care Process Process Model Model KEY POINTS Acute onset, severe hypertension in Obstetrics defined: • SBP ≥160 and/or • DBP ≥110 • And persistent for 15 minutes or longer. • Treatment with first line agents occurs withing 30- 60 minutes of confirmed severe hypertension • First line antihypertensives include: • IV labetalol • IV hydralazine or WHAT’S INSIDE: • Oral, short acting nifedipine (When IV access is Topic: Page not present) Key Points……………….………………….........2 • Minimum intervals between antihypertensives are ACOG Definitions………..…………...............3 different: Blood Pressure Measurement.............4-6 • IV hydralazine and nifedipine Preeclampsia Early Recognition Tool….7 • 20 minutes or greater intervals Nursing Assessment..…..……...….…….8-9 • IV labetalol Hypertensive Emergency……………….…10 • 10 minutes or greater interval Antenatal Management……………….…..11 Medications……………………………….…….12 • Cardiac monitoring is not required; Can be Delivery Indications………………………….13