The Disappearing Native Mammals of Northern Australia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

BANDICOTA INDICA, the BANDICOOT RAT 3.1 The
CHAPTER THREE BANDICOTA INDICA, THE BANDICOOT RAT 3.1 The Living Animal 3.1.1 Zoology Rats and mice (family Muridae) are the most common and well-known rodents, not only of the fi elds, cultivated areas, gardens, and storage places but especially so of the houses. Though there are many genera and species, their general appearance is pretty the same. Rats are on average twice as large as mice (see Chapter 31). The bandicoot is the largest rat on the Indian subcontinent, with a body and head length of 30–40 cm and an equally long tail; this is twice as large as the black rat or common house rat (see section 3.1.2 below). This large size immediately distinguishes the bandicoot from other rats. Bandicoots have a robust form, a rounded head, large rounded or oval ears, and a short, broad muzzle. Their long and naked scaly tail is typical of practically all rats and mice. Bandicoots erect their piles of long hairs and grunt when excited. Bandicoots are found practically on the whole of the subcontinent from the Himalayas to Cape Comorin, including Sri Lanka, but they are not found in the deserts and the semi-arid zones of north-west India. Here, they are replaced by a related species, the short-tailed bandicoot (see section 3.1.2 below). The bandicoot is essentially parasitic on man, living in or about human dwellings. They cause a lot of damage to grounds and fl oorings because of their burrowing habits; they also dig tunnels through bricks and masonry. -

Biodiversity Plan for the South East of South Australia 1999
SUMMARY Biodiversity Plan for the South East of South Australia 1999 rks & W Pa i Department for Environment ld l a l i f n e o i t Heritage and Aboriginal Affairs a N South Government of South Australia Australia AUTHORS Tim Croft (National Parks & Wildlife SA) Georgina House (QED) Alison Oppermann (National Parks & Wildlife SA) Ann Shaw Rungie (QED) Tatia Zubrinich (PPK Environment & Infrastructure Pty Ltd) CARTOGRAPHY AND DESIGN National Parks & Wildlife SA (Cover) Geographic Analysis and Research Unit, Planning SA Pierris Kahrimanis PPK Environment & Infrastructure Pty Ltd ACKNOWLEDGEMENTS The authors are grateful to Professor Hugh Possingham, the Nature Conservation Society, and the South Australian Farmers Federation in providing the stimulus for the Biodiversity Planning Program and for their ongoing support and involvement Dr Bob Inns and Professor Possingham have also contributed significantly towards the information and design of the South East Biodiversity Plan. We also thank members of the South East community who have provided direction and input into the plan through consultation and participation in workshops © Department for Environment, Heritage and Aboriginal Affairs, 1999 ISBN 0 7308 5863 4 Cover Photographs (top to bottom) Lowan phebalium (Phebalium lowanense) Photo: D.N. Kraehenbuehl Swamp Skink (Egernia coventryi) Photo: J. van Weenen Jaffray Swamp Photo: G. Carpenter Little Pygmy Possum (Cercartetus lepidus) Photo: P. Aitken Red-necked Wallaby (Macropus rufogriseus) Photo: P. Canty 2 diversity Plan for the South East of South Australia — Summary Foreword The conservation of our natural biodiversity is essential for the functioning of natural systems. Aside from the intrinsic importance of conserving the diversity of species many of South Australia's economic activities are based on the sustainable use, conservation and management of biodiversity. -

Macropod Herpesviruses Dec 2013
Herpesviruses and macropods Fact sheet Introductory statement Despite the widespread distribution of herpesviruses across a large range of macropod species there is a lack of detailed knowledge about these viruses and the effects they have on their hosts. While they have been associated with significant mortality events infections are usually benign, producing no or minimal clinical effects in their adapted hosts. With increasing emphasis being placed on captive breeding, reintroduction and translocation programs there is a greater likelihood that these viruses will be introduced into naïve macropod populations. The effects and implications of this type of viral movement are unclear. Aetiology Herpesviruses are enveloped DNA viruses that range in size from 120 to 250nm. The family Herpesviridae is divided into three subfamilies. Alphaherpesviruses have a moderately wide host range, rapid growth, lyse infected cells and have the capacity to establish latent infections primarily, but not exclusively, in nerve ganglia. Betaherpesviruses have a more restricted host range, a long replicative cycle, the capacity to cause infected cells to enlarge and the ability to form latent infections in secretory glands, lymphoreticular tissue, kidneys and other tissues. Gammaherpesviruses have a narrow host range, replicate in lymphoid cells, may induce neoplasia in infected cells and form latent infections in lymphoid tissue (Lachlan and Dubovi 2011, Roizman and Pellet 2001). There have been five herpesvirus species isolated from macropods, three alphaherpesviruses termed Macropodid Herpesvirus 1 (MaHV1), Macropodid Herpesvirus 2 (MaHV2), and Macropodid Herpesvirus 4 (MaHV4) and two gammaherpesviruses including Macropodid Herpesvirus 3 (MaHV3), and a currently unclassified novel gammaherpesvirus detected in swamp wallabies (Wallabia bicolor) (Callinan and Kefford 1981, Finnie et al. -

Checklist of the Mammals of Indonesia
CHECKLIST OF THE MAMMALS OF INDONESIA Scientific, English, Indonesia Name and Distribution Area Table in Indonesia Including CITES, IUCN and Indonesian Category for Conservation i ii CHECKLIST OF THE MAMMALS OF INDONESIA Scientific, English, Indonesia Name and Distribution Area Table in Indonesia Including CITES, IUCN and Indonesian Category for Conservation By Ibnu Maryanto Maharadatunkamsi Anang Setiawan Achmadi Sigit Wiantoro Eko Sulistyadi Masaaki Yoneda Agustinus Suyanto Jito Sugardjito RESEARCH CENTER FOR BIOLOGY INDONESIAN INSTITUTE OF SCIENCES (LIPI) iii © 2019 RESEARCH CENTER FOR BIOLOGY, INDONESIAN INSTITUTE OF SCIENCES (LIPI) Cataloging in Publication Data. CHECKLIST OF THE MAMMALS OF INDONESIA: Scientific, English, Indonesia Name and Distribution Area Table in Indonesia Including CITES, IUCN and Indonesian Category for Conservation/ Ibnu Maryanto, Maharadatunkamsi, Anang Setiawan Achmadi, Sigit Wiantoro, Eko Sulistyadi, Masaaki Yoneda, Agustinus Suyanto, & Jito Sugardjito. ix+ 66 pp; 21 x 29,7 cm ISBN: 978-979-579-108-9 1. Checklist of mammals 2. Indonesia Cover Desain : Eko Harsono Photo : I. Maryanto Third Edition : December 2019 Published by: RESEARCH CENTER FOR BIOLOGY, INDONESIAN INSTITUTE OF SCIENCES (LIPI). Jl Raya Jakarta-Bogor, Km 46, Cibinong, Bogor, Jawa Barat 16911 Telp: 021-87907604/87907636; Fax: 021-87907612 Email: [email protected] . iv PREFACE TO THIRD EDITION This book is a third edition of checklist of the Mammals of Indonesia. The new edition provides remarkable information in several ways compare to the first and second editions, the remarks column contain the abbreviation of the specific island distributions, synonym and specific location. Thus, in this edition we are also corrected the distribution of some species including some new additional species in accordance with the discovery of new species in Indonesia. -

Platypus Collins, L.R
AUSTRALIAN MAMMALS BIOLOGY AND CAPTIVE MANAGEMENT Stephen Jackson © CSIRO 2003 All rights reserved. Except under the conditions described in the Australian Copyright Act 1968 and subsequent amendments, no part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, duplicating or otherwise, without the prior permission of the copyright owner. Contact CSIRO PUBLISHING for all permission requests. National Library of Australia Cataloguing-in-Publication entry Jackson, Stephen M. Australian mammals: Biology and captive management Bibliography. ISBN 0 643 06635 7. 1. Mammals – Australia. 2. Captive mammals. I. Title. 599.0994 Available from CSIRO PUBLISHING 150 Oxford Street (PO Box 1139) Collingwood VIC 3066 Australia Telephone: +61 3 9662 7666 Local call: 1300 788 000 (Australia only) Fax: +61 3 9662 7555 Email: [email protected] Web site: www.publish.csiro.au Cover photos courtesy Stephen Jackson, Esther Beaton and Nick Alexander Set in Minion and Optima Cover and text design by James Kelly Typeset by Desktop Concepts Pty Ltd Printed in Australia by Ligare REFERENCES reserved. Chapter 1 – Platypus Collins, L.R. (1973) Monotremes and Marsupials: A Reference for Zoological Institutions. Smithsonian Institution Press, rights Austin, M.A. (1997) A Practical Guide to the Successful Washington. All Handrearing of Tasmanian Marsupials. Regal Publications, Collins, G.H., Whittington, R.J. & Canfield, P.J. (1986) Melbourne. Theileria ornithorhynchi Mackerras, 1959 in the platypus, 2003. Beaven, M. (1997) Hand rearing of a juvenile platypus. Ornithorhynchus anatinus (Shaw). Journal of Wildlife Proceedings of the ASZK/ARAZPA Conference. 16–20 March. -

Post-Release Monitoring of Western Grey Kangaroos (Macropus Fuliginosus) Relocated from an Urban Development Site
animals Article Post-Release Monitoring of Western Grey Kangaroos (Macropus fuliginosus) Relocated from an Urban Development Site Mark Cowan 1,* , Mark Blythman 1, John Angus 1 and Lesley Gibson 2 1 Biodiversity and Conservation Science, Department of Biodiversity, Conservation and Attractions, Wildlife Research Centre, Woodvale, WA 6026, Australia; [email protected] (M.B.); [email protected] (J.A.) 2 Biodiversity and Conservation Science, Department of Biodiversity, Conservation and Attractions, Kensington, WA 6151, Australia; [email protected] * Correspondence: [email protected]; Tel.: +61-8-9405-5141 Received: 31 August 2020; Accepted: 5 October 2020; Published: 19 October 2020 Simple Summary: As a result of urban development, 122 western grey kangaroos (Macropus fuliginosus) were relocated from the outskirts of Perth, Western Australia, to a nearby forest. Tracking collars were fitted to 67 of the kangaroos to monitor survival rates and movement patterns over 12 months. Spotlighting and camera traps were used as a secondary monitoring technique particularly for those kangaroos without collars. The survival rate of kangaroos was poor, with an estimated 80% dying within the first month following relocation and only six collared kangaroos surviving for up to 12 months. This result implicates stress associated with the capture, handling, and transport of animals as the likely cause. The unexpected rapid rate of mortality emphasises the importance of minimising stress when undertaking animal relocations. Abstract: The expansion of urban areas and associated clearing of habitat can have severe consequences for native wildlife. One option for managing wildlife in these situations is to relocate them. -

Calaby References
Abbott, I.J. (1974). Natural history of Curtis Island, Bass Strait. 5. Birds, with some notes on mammal trapping. Papers and Proceedings of the Royal Society of Tasmania 107: 171–74. General; Rodents; Abbott, I. (1978). Seabird islands No. 56 Michaelmas Island, King George Sound, Western Australia. Corella 2: 26–27. (Records rabbit and Rattus fuscipes). General; Rodents; Lagomorphs; Abbott, I. (1981). Seabird Islands No. 106 Mondrain Island, Archipelago of the Recherche, Western Australia. Corella 5: 60–61. (Records bush-rat and rock-wallaby). General; Rodents; Abbott, I. and Watson, J.R. (1978). The soils, flora, vegetation and vertebrate fauna of Chatham Island, Western Australia. Journal of the Royal Society of Western Australia 60: 65–70. (Only mammal is Rattus fuscipes). General; Rodents; Adams, D.B. (1980). Motivational systems of agonistic behaviour in muroid rodents: a comparative review and neural model. Aggressive Behavior 6: 295–346. Rodents; Ahern, L.D., Brown, P.R., Robertson, P. and Seebeck, J.H. (1985). Application of a taxon priority system to some Victorian vertebrate fauna. Fisheries and Wildlife Service, Victoria, Arthur Rylah Institute of Environmental Research Technical Report No. 32: 1–48. General; Marsupials; Bats; Rodents; Whales; Land Carnivores; Aitken, P. (1968). Observations on Notomys fuscus (Wood Jones) (Muridae-Pseudomyinae) with notes on a new synonym. South Australian Naturalist 43: 37–45. Rodents; Aitken, P.F. (1969). The mammals of the Flinders Ranges. Pp. 255–356 in Corbett, D.W.P. (ed.) The natural history of the Flinders Ranges. Libraries Board of South Australia : Adelaide. (Gives descriptions and notes on the echidna, marsupials, murids, and bats recorded for the Flinders Ranges; also deals with the introduced mammals, including the dingo). -

The Mahogany Glider
ISSUE #3 – WINTER 2019 THE MAHOGANY GLIDER The mahogany glider is one of Australia’s most threatened mammals and Queensland’s only listed endangered glider species. Named for its mahogany-brown belly, this graceful glider has two folds of skin, called a patagium, which stretch A mahogany glider, Petaurus gracilis, venturing out at the Hidden Vale Wildlife Centre. between the front and rear legs. These act as a ‘parachute’ enabling the animal to glide distances of and the Hidden Vale Wildlife Centre is manipulate their patagium in order to 30 metres in open woodland habitat. one of just a few locations authorised land safely. Their long tail is used for mid-air for breeding. Breeding is undertaken The Centre recently received funding stabilisation. as an insurance against extinction. to purchase additional structures The Centre regularly exchanges They look similar to sugar or squirrel including climbing ropes and nets, gliders with other authorised gliders, however mahogany gliders ladders and ‘unstable’ branches to locations to ensure genetic are much larger. They are nocturnal, simulate the movement of small trees. diversity in captive populations. elusive and silent, making research on We are also planning to introduce free-ranging animals very difficult. We encourage our mahogany gliders a range of glider-suitable feeding to develop and display their normal Mahogany gliders appear to be enrichment, such as whole fruits, range of behaviours. That is why we monogamous and will actively mark puzzle-feeders, or feed in a hanging have set up one half of their enclosure and defend their territory. They use log. We hope that our gliders will not with climbing structures in the form hollows in large eucalypt trees, lined only breed well, but will also become of ‘stable’ branches, while the right with a thick mat of leaves, as dens. -
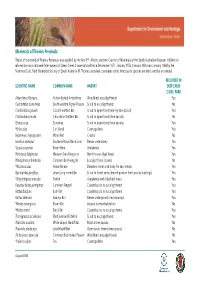
Mammals of Fleurieu Peninsula This List of Mammals of Fleurieu Peninsula Was Applied by the Late P.F
Mammals of Fleurieu Peninsula This list of mammals of Fleurieu Peninsula was applied by the late P.F. Aitken, onetime Curator of Mammals at the South Australian Museum. Additional information was obtained from surveys of Deep Creek Conservation Park in December 1971, January 1972, January 1980 and January 1984 by the Mammal Club, Field Naturalists Society of South Australia (R. Thomas, personal communication). Introduced species are indicated by an asterisk. RECORDED IN SCIENTIFIC NAME COMMON NAME HABITAT DEEP CREEK CONS. PARK Antechinus Flavipes Yellow-footed Antechinus Woodland, eucalypt forest Yes Cercartetus concinnus South western Pigmy Possum Scrub to eucalypt forest No Chalinolobus gouldii Gould's wattled Bat Scrub to open-forest (mainly tree spouts) Yes Chalinolobus moria Chocolate Wattled Bat Scrub to open-forest (tree spouts) No Eptesicus sp. Eptesicus Scrub to open-forest (tree spouts) Yes *Felis calus Cat (feral) Cosmopolitan Yes Hydromys chrysogaster Water Rat Creeks No Isoodon obesulus Southern Brown Bandicoot Dense understorey Yes *Lepus capensis Brown Hare Grasslands Yes Macropus fuliginosus Western Grey Kangaroo Heath to eucalypt forest Yes Miniopterus schreibersii Common Bent-wing Ba Eucalypt forest (caves) No *Mus musculus House Mouse Disturbed areas and early fire succession Yes Nyctophilus geoffroy Lesser Long-eared Bat Scrub to forest, semi cleared pasture (tree spouts, buildings) Yes *Oryctolagus cuniculus Rabbit Grasslands and disturbed areas Yes Pseudocheirus peregrinus Common Ringtail Coastal scrub to eucalypt -

Natural History of the Eutheria
FAUNA of AUSTRALIA 35. NATURAL HISTORY OF THE EUTHERIA P. J. JARMAN, A. K. LEE & L. S. HALL (with thanks for help to J.H. Calaby, G.M. McKay & M.M. Bryden) 1 35. NATURAL HISTORY OF THE EUTHERIA 2 35. NATURAL HISTORY OF THE EUTHERIA INTRODUCTION Unlike the Australian metatherian species which are all indigenous, terrestrial and non-flying, the eutherians now found in the continent are a mixture of indigenous and exotic species. Among the latter are some intentionally and some accidentally introduced species, and marine as well as terrestrial and flying as well as non-flying species are abundantly represented. All the habitats occupied by metatherians also are occupied by eutherians. Eutherians more than cover the metatherian weight range of 5 g–100 kg, but the largest terrestrial eutherians (which are introduced species) are an order of magnitude heavier than the largest extant metatherians. Before the arrival of dingoes 4000 years ago, however, none of the indigenous fully terrestrial eutherians weighed more than a kilogram, while most of the exotic species weigh more than that. The eutherians now represented in Australia are very diverse. They fall into major suites of species: Muridae; Chiroptera; marine mammals (whales, seals and dugong); introduced carnivores (Canidae and Felidae); introduced Leporidae (hares and rabbits); and introduced ungulates (Perissodactyla and Artiodactyla). In this chapter an attempt is made to compare and contrast the main features of the natural histories of these suites of species and, where appropriate, to comment on their resemblance to or difference from the metatherians. NATURAL HISTORY Ecology Diet. The native rodents are predominantly omnivorous. -

Impact of Fox Baiting on Tiger Quoll Populations Project ID: 00016505
Impact of fox baiting on tiger quoll populations Project ID: 00016505 Final Report to Environment Australia and The New South Wales National Parks and Wildlife Service Gerhard Körtner and Shaan Gresser Copyright G. Körtner Executive Summary: The NSW Threat Abatement Plan for Predation by the Red Fox (TAP) identifies foxes as a major threat to the survival of many native mammals. The plan recommends baiting with compound 1080 (sodium monofluoroacetate) because it appears to be the most effective fox control measure. However, the plan also recognises the risk for tiger quolls as a non-target species. Although the actual impact of 1080 fox baiting on tiger quoll populations has not been assessed, this assumed risk has resulted in restrictions on the use of 1080 which render fox baiting programs labour intensive and expensive and which may compromise the effectiveness of the fox control. The aim of this project is to determine whether these precautions are necessary by measuring tiger quoll mortality during fox baiting programs using 1080. The project has been identified as a priority action (Obj. 2, action 5) of the TAP. Three experiments were conducted in north-east NSW between June 2000 and December 2001. Overall 78 quolls were trapped and 56 of those were fitted with mortality radio-transmitters. Baiting procedure followed Best Practice Guidelines (TAP) except that there was no free-feeding and baits were only surface buried. These modifications aimed to increase the exposure of quolls to bait. 1080 baits (3 mg / bait; Foxoff®) incorporating the bait marker Rhodamine B were deployed for 10 days along existing trails. -

Reproductionreview
REPRODUCTIONREVIEW Wombat reproduction (Marsupialia; Vombatidae): an update and future directions for the development of artificial breeding technology Lindsay A Hogan1, Tina Janssen2 and Stephen D Johnston1,2 1Wildlife Biology Unit, Faculty of Science, School of Agricultural and Food Sciences, The University of Queensland, Gatton 4343, Queensland, Australia and 2Australian Animals Care and Education, Mt Larcom 4695, Queensland, Australia Correspondence should be addressed to L A Hogan; Email: [email protected] Abstract This review provides an update on what is currently known about wombat reproductive biology and reports on attempts made to manipulate and/or enhance wombat reproduction as part of the development of artificial reproductive technology (ART) in this taxon. Over the last decade, the logistical difficulties associated with monitoring a nocturnal and semi-fossorial species have largely been overcome, enabling new features of wombat physiology and behaviour to be elucidated. Despite this progress, captive propagation rates are still poor and there are areas of wombat reproductive biology that still require attention, e.g. further characterisation of the oestrous cycle and oestrus. Numerous advances in the use of ART have also been recently developed in the Vombatidae but despite this research, practical methods of manipulating wombat reproduction for the purposes of obtaining research material or for artificial breeding are not yet available. Improvement of the propagation, genetic diversity and management of wombat populations requires a thorough understanding of Vombatidae reproduction. While semen collection and cryopreservation in wombats is fairly straightforward there is currently an inability to detect, induce or synchronise oestrus/ovulation and this is an impeding progress in the development of artificial insemination in this taxon.