Food Labeling Guide 4
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Country-Of-Origin Labeling for Foods
Country-of-Origin Labeling for Foods Remy Jurenas Specialist in Agricultural Policy July 15, 2010 Congressional Research Service 7-5700 www.crs.gov RS22955 CRS Report for Congress Prepared for Members and Committees of Congress Country-of-Origin Labeling for Foods Summary Many retail food stores are now required to inform consumers about the country of origin of fresh fruits and vegetables, seafood, peanuts, pecans, macadamia nuts, ginseng, and ground and muscle cuts of beef, pork, lamb, chicken, and goat. The rules are required by the 2002 farm bill (P.L. 107- 171) as amended by the 2008 farm bill (P.L. 110-246). Other U.S. laws have required such labeling, but only for imported food products already pre-packaged for consumers. Both the authorization and implementation of country-of-origin labeling (COOL) by the U.S. Department of Agriculture’s Agricultural Marketing Service have not been without controversy. Much attention has focused on the labeling rules that now apply to meat and meat product imports. A number of leading agricultural and food industry groups continue to oppose COOL as costly and unnecessary. They and some major food and livestock exporters to the United States (e.g., Canada and Mexico) also view the new requirement as trade-distorting. Others, including some cattle and consumer groups, maintain that Americans want and deserve to know the origin of their foods, and that many U.S. trading partners have their own, equally restrictive import labeling requirements. Obama Administration officials announced in February 2009 that they would allow the final rule on COOL, published just before the end of the Bush Administration on January 15, 2009, to take effect as planned on March 16, 2009. -

Measuring the Value of Information in Nuval Shelf Nutrition Label Feifei Liang, North Carolina State University Chen Zhen, Unive
Measuring the Value of Information in NuVal Shelf Nutrition Label Feifei Liang, North Carolina State University Chen Zhen, University of Georgia Xiaoyong Zheng, North Carolina State University July 2017 Abstract The objective of this study is to estimate the value of information provided by the NuVal shelf nutrition label ―a prominent example of a new interpretive approach to front-of-package nutrition labeling that provides a multiple-level nutrition symbol. The rollout of NuVal at a supermarket in a Midwest town allowed us to estimate a mixed logit demand model for yogurt using household and barcode-level scanner data. We found the NuVal label promoted purchases of healthier yogurts. The average value of NuVal information in the yogurt category is estimated to be about 3% of total expenditures on yogurt. There is evidence suggesting that households who made less use of conventional nutrition labels prior to NuVal rollout may stand to benefit more from NuVal labels. Overall, our results indicate that there is a significant willingness to pay, on the part of consumers, for a universal adoption of an interpretive multiple-level shelf or front-of-package nutrition symbol. Acknowledgment: This research was supported by USDA-NIFA Hatch Grants 02375 and 02598 and a grant from Healthy Eating Research, a national program of the Robert Wood Johnson Foundation. The diet for the majority of the U.S. population does not meet the Dietary Guidelines for Americans (DGA) (USDA and DHHS, 2010). Per capita caloric intake from solid fats and added sugars exceeds the recommended limit by 180%, the highest percentage of foods and food components consumed excessively by Americans, followed by refined grains (100%) and sodium (49%) (USDA and DHHS, 2010). -

Information on Nutrition and Health Claims
Information on Nutrition and Health Claims April 2021 High in Vitamin C Rich in calcium Contains omega 3 With no added sugars Fat-free Cholesterol free Low energyInformation Energy-reduced on Energy-free Low fat Fat-free Low saturated fat Source of omega-3 fattyNutrition acids andHigh omega-3 fatty acids High monounsaturated fat High polyunsaturated fat High unsaturatedHealth Claims fat Saturated fat-free Low sugars Sugars-free With no added sugars Low sodium/salt Very low sodium/salt Sodium-free/salt-free Source of fibre High fibre Source April 2021 of protein High protein Source of vitamins and minerals Contains nutrients Increased nutrients Published by: Food Safety Authority of Ireland The Exchange, George’s Dock, IFSC, Dublin 1, D01 P2V6 T +353 1 817 1300 E [email protected] www.fsai.ie ISBN 1-904465-69-2 © FSAI 2019 Applications for reproduction should be made to the FSAI Information Unit. Contents Background 2 Legislation Referred to in this Document 2 Nutrition and Health Claims Overview 2 Nutrition Claims 4 Making ‘source-of’ Claims on Food Products 10 Health Claims 13 Nutrition Labelling Requirements under Regulation EU No 1169/2011 (FIC) 19 Mandatory Nutrition Labelling 20 Front of Pack (FoP) Nutrition Labelling (Voluntary) 25 Sample Labels 26 Annex 1. How I Can Make Permitted Nutrition and Health Claims on My Food Product – Practical Examples 29 page 1 Food Safety Authority of Ireland Background This document is guidance information on the regulation of nutrition and health claims in Ireland*. The document provides an overview of the: – Nutrition and health claims legislation – Nutrition labelling requirements for food products bearing authorised nutrition and health claims Legislation Referred to in this Document Throughout the document where guidance is given on aspects of the legislation, a reference to the relevant articles and legislation is included. -

How to Make a Smell Training Kit July 3 2019
Some frequently asked questions Q. How much oil do I need in the jar? A. You only need enough to saturate the paper disc. Any more than that is just a waste of the oil. Q. I can’t smell anything! Have I done it wrong? A. Probably not. If you’ve followed the directions, your jars should be plenty “smelly”. The saturated disc, kept in the closed space with the cap on the jar, creates a really strong smell. If you are not smelling it now, give it time. Q. Can I put my nose all the way into the jar? A. That is not recommended. Keep the tip of your nose out of the jar. Q. What if I want to reuse the jar, but with different oils? A. You can do this, but you need to give the jars a really Smell Training Kits good clean with hot water and soap. Let them dry thoroughly. The lid will smell like the previous oil (not great, but you could improvise and remove the inside of the cap, which is made of white, plastic coated paper). Then cut yourself some new watercolour paper discs and make up the new jars. How to make your own Q. Can I use cotton pads inside the jars? A. Cotton pads are not recommended. They make a great place for bacteria to collect. Watercolour paper is absorbent, but does not harbour bacteria. Contact details E: [email protected] • W: abscent.org © AbScent is a charity registered in England and Wales No. 1183468• • Registered Office: 14 London Street, Andover, Hampshire SP10 2PA © AbScent 2019 Making your own kit is easy Just follow these simple steps. -

The Facts About Plastic Bags: Recyclable, Affordable, and Convenient
THE FACTS ABOUT PLASTIC BAGS: RECYCLABLE, AFFORDABLE, AND CONVENIENT Plastic bags are 100% recyclable, reusable, made from natural gas, not oil, and a sustainable choice for consumers, communities and businesses. What’s more, the plastic bag manufacturing and recycling industry is a uniquely American industry that employs more than 30,000 Americans in 349 plants across the country, including more than 1,000 people in Washington state. Bans and taxes on plastic bags are misguided policies that don’t make sense. They don’t help the environment, force less sustainable options, threaten local manufacturing jobs and raise grocery costs for consumers. Instead of banning a reusable, 100% recyclable, American-made product, recycling solutions can help reduce litter, give consumers a choice, and protect American jobs. Plastic grocery bags are the best checkout option for our environment On a per bag basis, plastic bags are more resource efficient, reduce landfill waste and generate fewer greenhouse gas emissions. o They take up a lot less space in a landfill: 1,000 plastic bags weigh 13 pounds; 1,000 paper bags weigh 114 pounds.i o They generate 80 % less waste than paper bags.ii American plastic bags are made from natural gas, NOT oil. In the U.S., 85 percent of the raw material used to make plastic bags is produced from natural gas.iii Recycled plastic bags are used to make new plastic bags and building products, such as backyard decks, playground equipment, and fences. Bans haven’t worked in other places, and don’t protect the environment A ban would make no difference in litter reduction since plastic bags only make up a tiny fraction (less than 0.5 %) of the U.S. -
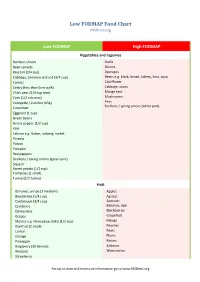
Low FODMAP Food Chart Ibsdiets.Org
Low FODMAP Food Chart IBSDiets.org Low FODMAP High FODMAP Vegetables and Legumes Bamboo shoots Garlic Bean sprouts Onions Broccoli (3/4 cup) Aparagus Cabbage, common and red (3/4 cup) Beans e.g. black, broad, kidney, lima, soya Carrots Cauliflower Celery (less than 5cm stalk) Cabbage, savoy Chick peas (1/4 cup max) Mange tout Corn (1/2 cob max) Mushrooms Courgette / Zucchini (65g) Peas Scallions / spring onions (white part) Cucumber Eggplant (1 cup) Green beans Green pepper (1/2 cup) Kale Lettuce e.g. Butter, iceberg, rocket Parsnip Potato Pumpkin Red peppers Scallions / spring onions (green part) Squash Sweet potato (1/2 cup) Tomatoes (1 small) Turnip (1/2 turnip) Fruit Bananas, unripe (1 medium) Apples Blueberries (1/4 cup) Apricot Cantaloupe (3/4 cup) Avocado Cranberry Bananas, ripe Clementine Blackberries Grapes Grapefruit Melons e.g. Honeydew, Galia (1/2 cup) Mango Kiwifruit (2 small) Peaches Lemon Pears Orange Plums Pineapple Raisins Raspberry (30 berries) Sultanas Rhubarb Watermelon Strawberry For up to date and extensive information go to www.IBSDiets.org Meat and Substitutes Beef Sausages (check ingredients) Chicken Processed meat (check ingredients) Lamb Pork Quorn mince Cold cuts e.g. Ham and turkey breast Breads, Cereals, Grains and Pasta Oats Barley Quinoa Bran Gluten free foods e.g. breads, pasta Cous cous Savory biscuits Gnocchi Buckwheat Granola Chips / crisps (plain) Muesli Cornflour Muffins Oatmeal (1/2 cup max) Rye Popcorn Semolina Pretzels Spelt Rice e.g. Basmati, brown, white Wheat foods e.g. Bread, cereal, pasta -

Elemental and Semi-Elemental Formulas: Are They Superior to Polymeric Formulas?
NUTRITION ISSUES IN GASTROENTEROLOGY, SERIES #34 Carol Rees Parrish, R.D., M.S., Series Editor Elemental and Semi-Elemental Formulas: Are They Superior to Polymeric Formulas? Diklar Makola Nutritional support, utilizing enteral nutrition formulas, is an integral part of the pri- mary and/or adjunctive management of gastrointestinal and other disorders with nutritional consequences. Four major types of enteral nutrition formulas exist includ- ing: elemental and semi-elemental, standard or polymeric, disease-specific and immune-enhancing. Although they are much more expensive, elemental and semi- elemental formulas are purported to be superior to polymeric or standard formulas in certain patient populations. The aim of this article is to evaluate whether this claim is supported by the literature and to ultimately show that except for a very few indica- tions, polymeric formulas are just as effective as elemental formulas in the majority of patients with gastrointestinal disorders. INTRODUCTION ease, facilitate “pancreatic rest” in pancreatitis and pre- he provision of nutritional support is an essential vent nutritional depletion that accompanies many GI part of the primary and adjunctive management of tract diseases. The factors leading to nutritional deple- T tion include: 1) impaired absorption of nutrients; many gastrointestinal (GI) disorders such as Crohn’s disease, cystic fibrosis, pancreatitis, head and 2) inadequate intake due to anorexia; 3) dietary restric- neck cancer, cerebrovascular accidents, etc. Nutritional tions; 4) increased intestinal losses; and 5) an increase in support can be used to induce remission in Crohn’s dis- nutritional demand that accompanies many catabolic states. Nutritional support can be provided by using Diklar Makola, M.D., M.P.H., Ph.D., Gastroenterology either total parenteral nutrition (PN) or total enteral Fellow, University of Virginia Health System, Digestive nutrition (EN), however EN when compared to PN has Health Center of Excellence, Charlottesville, VA. -

Guide for Labeling Consumer Package by Weight, Volume, Count, Or Measure (Length, Area Or Thickness)
NIST Special Publication 1020 Guide for Labeling Consumer Package by Weight, Volume, Count, or Measure (length, area or thickness) Editors: David Sefcik Lisa Warfield This publication is available free of charge from: https://doi.org/10.6028/NIST.SP.1020 NIST Special Publication 1020 Guide for Labeling Consumer Package by Weight, Volume, Count, or Measure (length, area or thickness) Editors: David Sefcik Lisa Warfield Dr. Douglas Olson, Chief Office of Weights and Measures Physical Measurement Laboratory This publication is available free of charge from: https://doi.org/10.6028/NIST.SP.1020 June 2020 NIST SP 1020 supersedes all previous editions U.S. Department of Commerce Wilbur L. Ross, Jr., Secretary National Institute of Standards and Technology Walter Copan, NIST Director and Undersecretary of Commerce for Standards and Technology Certain commercial entities, equipment, or materials may be identified in this document in order to describe an experimental procedure or concept adequately. Such identification is not intended to imply recommendation or endorsement by the National Institute of Standards and Technology, nor is it intended to imply that the entities, materials, or equipment are necessarily the best available for the purpose. National Institute of Standards and Technology Special Publication 1020 Natl. Inst. Stand. Technol. Spec. Publ. 1020, 40 pages (June 2020) This publication is available free of charge from: https://doi.org/10.6028/NIST.SP.1020 Foreword This document, “Guide for Labeling Consumer Packages by Weight, Volume, Count, or Measure (length, area, or thickness),” is based on the Uniform Packaging and Labeling Regulation (UPLR) in National Institute of Standards and Technology Handbook 130, “Uniform Laws and Regulation in the Areas of Legal Metrology and Fuel Quality.” It provides a summary of labeling requirements for consumer products and commodities sold by weight, volume, count, or measure. -

Boil in Bag Meals a Burner's Laziest and Easiest Option
Boil In Bag Meals A Burner’s laziest and easiest option. Boil‐in‐bag meals are a fantastic option for those of us that want a quick, nutritious home‐made meal on the playa with little‐to‐no waste. Here’s a quick run down of the boil‐in‐bag meal plan I executed in 2009 on the playa. The possibilities are limitless on these and you can tailor them to any diet, taste or hunger level. The basic boil‐in‐bag meal consists of 4 components… 1) A starch‐ rice, couscous or noodles 2) A protein‐ beef, chicken, meatballs, or tofu 3) An accompaniment‐ Veggies, “flavor profile items” (bacon, cherries, mint) and such 4) A sauce There are very specific ways to prepare each of these components so that they (and in turn, the dish) turn out as tasty and appetizing as possible… I will go into these in detail over the next few pages. First there are a few things you will need… a) Vacuum sealer with appropriate bags‐ I’m pretty sure this is the one I got for last year‐> http://www.amazon.com/Seal‐A‐Meal‐VS107‐Vacuum‐Food‐ Sealer/dp/B000KL5IJM/ref=sr_1_5?ie=UTF8&s=kitchen&qid=1279311399&sr= 1‐5 As far as bags go, there are individual 1‐quart bags that work well for single and double serving meals, and larger rolls of bags that you can cut to size and seal for larger meals or fruit/berries b) Large flat trays (aluminum disposable turkey roasting trays or flatter serving pans work best) to freeze the components and a fair amount of freezer space (Chest freezer works great) c) 2 gallon ziplock bags for freezing components d) A LOT of ice trays e) Coolers, dry ice and newspaper. -

879 Part 134—Country of Origin Marking
U.S. Customs and Border Protection, DHS; Treasury Pt. 134 removal or obliteration of the name, Subpart A—General Provisions mark, or trademark by reason of which 134.1 Definitions. the articles were seized. 134.2 Additional duties. (b) Copyright violations. Articles for- 134.3 Delivery withheld until marked and feited for violation of the copyright redelivery ordered. laws shall be destroyed. 134.4 Penalties for removal, defacement, or (c) Articles bearing a counterfeit trade- alteration of marking. mark. Merchandise forfeited for viola- Subpart B—Articles Subject to Marking tion of the trademark laws shall be de- stroyed, unless it is determined that 134.11 Country of origin marking required. the merchandise is not unsafe or a haz- 134.12 Foreign articles reshipped from a U.S. possession. ard to health and the Commissioner of 134.13 Imported articles repacked or manip- Customs or his designee has the writ- ulated. ten consent of the U.S. trademark 134.14 Articles usually combined. owner, in which case the Commissioner of Customs or his designee may dispose Subpart C—Marking of Containers or of the merchandise, after obliteration Holders of the trademark, where feasible, by: 134.21 Special marking. (1) Delivery to any Federal, State, or 134.22 General rules for marking of con- local government agency that, in the tainers or holders. opinion of the Commissioner or his des- 134.23 Containers or holders designed for or capable of reuse. ignee, has established a need for the 134.24 Containers or holders not designed merchandise; or for or capable of reuse. -

Health Claims in Food Advertising and Labeling
Chapter 11 Health Claims in Food Advertising and Labeling Disseminating Nutrition Information to Consumers Alan D. Mathios and Pauline Ippolito The question of how best to get evolving scientific evidence linking diet and disease to consumers has been much debated in policy cir- cles. At the core of this debate are widely varying presumptions about how effective food manufacturers are in reaching consumers com- pared with, or in addition to, government and other information sources, and about the best approaches for controlling misleading or deceptive claims. This chapter evaluates whether policy changes that took place in the mid-1980s, and allowed food manufacturers to explicitly link diet to disease risks in advertising and labeling, appear to have improved consumers food choices (the information hypothesis), or as many critics fear, to have confused consumers suf- ficiently to slow improvements in diet that would otherwise occur (the consumer confusion hypothesis). Introduction In the last 30 years, scientific understanding of the role of diet in chronic disease risks has improved significantly. In the United States, diet is now believed to be linked substantially to 4 of the top 10 causes of death, and diet-disease research is continuing at a rapid Mathios is Associate Professor, Department of Policy Analysis and Management, Cornell University; and Ippolito is Associate Director for Special Projects, Bureau of Economics, Federal Trade Commission. The majority of material in this chapter is drawn from several published studies by both authors. Health Claims • AIB-750 USDA/ERS • 189 pace. Individuals have much to gain from information that would allow them to incorporate this evolving science into basic dietary decisions. -

Getinge Pack Tyvek Sterilization Reels and Pouches
SAFETY DATA SHEET Getinge Pack Tyvek Sterilization Reels and Pouches 1.0 Product Product Name Getinge Pack Tyvek Sterilization Reels and Pouches and Company Product Code Tyvek Reel (TY)- Tyvek Pouch (TP) Identification Intended Use Tyvek Sterilization Reels and Pouches are intended to be used to enclose another medical devices that are to be sterilized by health care provider via related sterilization methods. Supplier PMS Tıbbi Cihazlar Teknolojisi Sanayi ve Ticaret A.Ş Identification Address Karaduvar Mah. Serbest Bölge 11. Cadde No: 46,Akdeniz, 33020 Mersin/TURKEY Phone +90 324 238 70 42 Fax +90 324 238 65 49 2.0 Hazards Tyvek material and PET/PE laminated film are not classified as dangerous according to EC directives and other international safety guidelines. Identification 3.0 Composition Information on Ingredients • Tyvek Material (1059B, 64.4 g/m² ) / Information on • Polyethylene Terephthalate / Polyethylene (PET/PE) Laminated Film Ingredients 12/50 microns • Printing inks, Indicator inks 4.0 First Aid Eye contact If molten material or dust contacts the eye, immediatly flush Measures with plenty of water for at least 15 minutes. If easy to do , remove contact lenses. Get medical attention immediately. Skin contact If contact with molten product occurs, treat as for thermal burn. Cool melted product on skin with plenty of water. Do not remove solidified product. If dust contacts the skin, immediately flush with plenty of water. Inhalation Remove patient to fresh air. Seek medical attention if necessary. Ingestion Material is not expected to be absorbed from the gastrointestinal tract. However, the printing chemicals such as printing inks and chemical indicator inks could be harmful.