University of Florida Thesis Or Dissertation Formatting
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guide to the Flora of the Carolinas, Virginia, and Georgia, Working Draft of 17 March 2004 -- LILIACEAE
Guide to the Flora of the Carolinas, Virginia, and Georgia, Working Draft of 17 March 2004 -- LILIACEAE LILIACEAE de Jussieu 1789 (Lily Family) (also see AGAVACEAE, ALLIACEAE, ALSTROEMERIACEAE, AMARYLLIDACEAE, ASPARAGACEAE, COLCHICACEAE, HEMEROCALLIDACEAE, HOSTACEAE, HYACINTHACEAE, HYPOXIDACEAE, MELANTHIACEAE, NARTHECIACEAE, RUSCACEAE, SMILACACEAE, THEMIDACEAE, TOFIELDIACEAE) As here interpreted narrowly, the Liliaceae constitutes about 11 genera and 550 species, of the Northern Hemisphere. There has been much recent investigation and re-interpretation of evidence regarding the upper-level taxonomy of the Liliales, with strong suggestions that the broad Liliaceae recognized by Cronquist (1981) is artificial and polyphyletic. Cronquist (1993) himself concurs, at least to a degree: "we still await a comprehensive reorganization of the lilies into several families more comparable to other recognized families of angiosperms." Dahlgren & Clifford (1982) and Dahlgren, Clifford, & Yeo (1985) synthesized an early phase in the modern revolution of monocot taxonomy. Since then, additional research, especially molecular (Duvall et al. 1993, Chase et al. 1993, Bogler & Simpson 1995, and many others), has strongly validated the general lines (and many details) of Dahlgren's arrangement. The most recent synthesis (Kubitzki 1998a) is followed as the basis for familial and generic taxonomy of the lilies and their relatives (see summary below). References: Angiosperm Phylogeny Group (1998, 2003); Tamura in Kubitzki (1998a). Our “liliaceous” genera (members of orders placed in the Lilianae) are therefore divided as shown below, largely following Kubitzki (1998a) and some more recent molecular analyses. ALISMATALES TOFIELDIACEAE: Pleea, Tofieldia. LILIALES ALSTROEMERIACEAE: Alstroemeria COLCHICACEAE: Colchicum, Uvularia. LILIACEAE: Clintonia, Erythronium, Lilium, Medeola, Prosartes, Streptopus, Tricyrtis, Tulipa. MELANTHIACEAE: Amianthium, Anticlea, Chamaelirium, Helonias, Melanthium, Schoenocaulon, Stenanthium, Veratrum, Toxicoscordion, Trillium, Xerophyllum, Zigadenus. -

State of New York City's Plants 2018
STATE OF NEW YORK CITY’S PLANTS 2018 Daniel Atha & Brian Boom © 2018 The New York Botanical Garden All rights reserved ISBN 978-0-89327-955-4 Center for Conservation Strategy The New York Botanical Garden 2900 Southern Boulevard Bronx, NY 10458 All photos NYBG staff Citation: Atha, D. and B. Boom. 2018. State of New York City’s Plants 2018. Center for Conservation Strategy. The New York Botanical Garden, Bronx, NY. 132 pp. STATE OF NEW YORK CITY’S PLANTS 2018 4 EXECUTIVE SUMMARY 6 INTRODUCTION 10 DOCUMENTING THE CITY’S PLANTS 10 The Flora of New York City 11 Rare Species 14 Focus on Specific Area 16 Botanical Spectacle: Summer Snow 18 CITIZEN SCIENCE 20 THREATS TO THE CITY’S PLANTS 24 NEW YORK STATE PROHIBITED AND REGULATED INVASIVE SPECIES FOUND IN NEW YORK CITY 26 LOOKING AHEAD 27 CONTRIBUTORS AND ACKNOWLEGMENTS 30 LITERATURE CITED 31 APPENDIX Checklist of the Spontaneous Vascular Plants of New York City 32 Ferns and Fern Allies 35 Gymnosperms 36 Nymphaeales and Magnoliids 37 Monocots 67 Dicots 3 EXECUTIVE SUMMARY This report, State of New York City’s Plants 2018, is the first rankings of rare, threatened, endangered, and extinct species of what is envisioned by the Center for Conservation Strategy known from New York City, and based on this compilation of The New York Botanical Garden as annual updates thirteen percent of the City’s flora is imperiled or extinct in New summarizing the status of the spontaneous plant species of the York City. five boroughs of New York City. This year’s report deals with the City’s vascular plants (ferns and fern allies, gymnosperms, We have begun the process of assessing conservation status and flowering plants), but in the future it is planned to phase in at the local level for all species. -

Epidendrum Secundum (Orchidaceae)
Plant Biology ISSN 1435-8603 RESEARCH PAPER Reproductive biology and pollination mechanisms of Epidendrum secundum (Orchidaceae). Floral variation: a consequence of natural hybridization? E. R. Pansarin & M. C. E. Amaral Departamento de Botaˆ nica, Instituto de Biologia, Universidade Estadual de Campinas, Sa˜ o Paulo, Brazil Keywords ABSTRACT Epidendroideae; Epidendrum; Laeliinae; Orchidaceae; pollination; reproductive biology. The phenology, flower morphology, pollination mechanism and reproductive biology of Epidendrum secundum were studied in a semi-deciduous forest at Correspondence the Serra do Japi (SJ), and in the Atlantic rain forest of Picinguaba, both E. R. Pansarin, Departamento de Biologia natural reserves in the State of Sa˜o Paulo, southeastern Brazil. E. secundum Aplicada, Universidade Estadual Paulista, flowers all year round, with a flowering peak between September and FCAV, 14884-900, Jaboticabal, SP, Brazil. January. This species is either a lithophytic or terrestrial herb in the SJ, E-mail: [email protected] whereas, in Picinguaba, it grows mainly in disturbed areas along roadsides. E. secundum is pollinated by several species of diurnal Lepidoptera at both Editor study sites. In Picinguaba, where E. secundum is sympatric with E. fulgens M. Ayasse and both share the same pollinators, pollen transference between these two species was recorded. E. secundum is self-compatible but pollinator-depen- Received: 25 March 2007; Accepted: 22 May dent. It is inter-compatible with E. fulgens, producing fertile seeds. In con- 2007 trast to the population of the SJ, in the Picinguaba region, floral morphology is quite variable among plants and some individuals present doi:10.1111/j.1438-8677.2007.00025.x flowers with characteristics in-between both sympatric species, suggesting that natural hybridization occasionally occurs. -

Orchids: 2017 Global Ex Situ Collections Assessment
Orchids: 2017 Global Ex situ Collections Assessment Botanic gardens collectively maintain one-third of Earth's plant diversity. Through their conservation, education, horticulture, and research activities, botanic gardens inspire millions of people each year about the importance of plants. Ophrys apifera (Bernard DuPon) Angraecum conchoglossum With one in five species facing extinction due to threats such (Scott Zona) as habitat loss, climate change, and invasive species, botanic garden ex situ collections serve a central purpose in preventing the loss of species and essential genetic diversity. To support the Global Strategy for Plant Conservation, botanic gardens create integrated conservation programs that utilize diverse partners and innovative techniques. As genetically diverse collections are developed, our collective global safety net against plant extinction is strengthened. Country-level distribution of orchids around the world (map data courtesy of Michael Harrington via ArcGIS) Left to right: Renanthera monachica (Dalton Holland Baptista ), Platanthera ciliaris (Wikimedia Commons Jhapeman) , Anacamptis boryi (Hans Stieglitz) and Paphiopedilum exul (Wikimedia Commons Orchi ). Orchids The diversity, stunning flowers, seductiveness, size, and ability to hybridize are all traits which make orchids extremely valuable Orchids (Orchidaceae) make up one of the largest plant families to collectors, florists, and horticulturists around the world. on Earth, comprising over 25,000 species and around 8% of all Over-collection of wild plants is a major cause of species flowering plants (Koopowitz, 2001). Orchids naturally occur on decline in the wild. Orchids are also very sensitive to nearly all continents and ecosystems on Earth, with high environmental changes, and increasing habitat loss and diversity found in tropical and subtropical regions. -

5. KALIDIUM Moquin-Tandon in Candolle, Prodr. 13(2): 46, 146
Flora of China 5: 355-356. 2003. 5. KALIDIUM Moquin-Tandon in Candolle, Prodr. 13(2): 46, 146. 1849. 盐爪爪属 yan zhua zhua shu Shrubs small, much branched; branches not jointed. Leaves alternate, terete or undeveloped, fleshy, basally decurrent. Inflorescence pedunculate, spicate. Flowers spirally arranged, (1 or)3 borne in axil of a fleshy bract, appearing sunken into fleshy rachis, without bractlets, bisexual. Perianth 4- or 5-lobed, spongy in fruit, flat on top surface. Stamens 2. Ovary ovoid; stigmas 2, papillate. Fruit a utricle, enclosed by perianth. Seed vertical, compressed; testa subleathery; embryo semi-annular; perisperm present. Five species: C and SW Asia, SE Europe; five species in China. 1a. Leaves 4–10 mm; spikes 3–4 mm in diam. .................................................................................................................... 1. K. foliatum 1b. Leaves less than 3 mm or undeveloped; spikes 1.5–3 mm in diam. 2a. Branchlets slender; flowers 1 per bract ..................................................................................................................... 5. K. gracile 2b. Branchlets stout; flowers 3 per bract. 3a. Leaves developed, 1–3 mm, ovate, adaxially curved, apex acute ............................................................... 2. K. cuspidatum 3b. Leaves undeveloped, tuberculate, less than 1 mm, apex obtuse. 4a. Plants 10–25 cm tall, branched from base; leaves of branchlets narrow and obconic at base ......... 3. K. schrenkianum 4b. Plants 20–70 cm tall, branched from middle; leaves of branchlets sheathing at base ............................ 4. K. caspicum 1. Kalidium foliatum (Pallas) Moquin-Tandon in Candolle, 1b. Leaves 1–1.5 mm; plants densely Prodr. 13(2): 147. 1849. branched .................................................... 2b. var. sinicum 盐爪爪 yan zhua zhua 2a. Kalidium cuspidatum var. cuspidatum Salicornia foliata Pallas, Reise Russ. Reich. 1: 482. -

Universidad Nacional De San Antonio Abad Del Cusco Facultad De Ciencias Escuela Profesional De Biología
Universidad Nacional de San Antonio Abad del Cusco Facultad de Ciencias Escuela Profesional de Biología MICROPROPAGACION DE ORQUÍDEAS A PARTIR DE SEMILLAS EN CONDICIONES DE CULTIVO IN VITRO Tesis Presentada por: Bachiller. Karina Flores Huisa Para Optar al Título Profesional de Biólogo Asesor: M. Sc. Máximo Américo Chacón Campana Cusco – Perú 2019 Dedicatoria 9 A Dios por protegerme cada día de mi vida. 9 A mis padres; Apolinaria y Francisco por darme la vida y ser un ejemplo a seguir. 9 A mis hermanos José Luis, Beatriz, Daniel, Juan Carlos, Milagros por sus palabras de aliento a seguir e inolvidables momentos compartidos en familia. 9 A mi mentor Dr. Luis Fernando Miranda Ángeles (ƚ) y familia por haber contribuido a mi formación profesional y todos sus consejos dados a lo largo de mi vida. Agradecimientos 9 A la Universidad Nacional de San Antonio Abad del Cusco por haberme formado en la Carrera de Ciencias Biológicas. 9 A la Universidad Nacional Agraria la Molina y al Instituto de Biotecnología (IBT) por haberme permitido continuar con mi formación profesional. 9 Un agradecimiento a mi asesor M.Sc. Blgo Máximo Américo Chacón Campana por su asesoramiento en la presente Tesis. 9 Al cuerpo docente de la Facultad de Ciencias, Escuela Profesional de Biología, por haber contribuido en mi formación profesional. 9 Mis agradecimientos a la Dra María E. Holgado Rojas, M.Sc Blga Gloria Calatayud Hermosa, al Blgo. Marcial Villafuerte Arriaga, por su ayuda en la colecta de orquídeas y sus valiosos aportes. 9 Al Blgo. Marco León Martínez por su donación de la especie de Epidendrum macrocarpum para la realización del trabajo. -

SOOS September 2012
SOUTHERN ONTARIO ORCHID SOCIETY NEWS September 2012, Volume 47, Issue 8 Web site: www.soos.ca ; Member of the Canadian Orchid Congress; Affiliated with the American Orchid Society, the Orchid Digest and the International Phalaenopsis Alliance. Membership: Annual Dues $30 per calendar year (January 1 to December 31 ). Surcharge $15 for newsletter by postal service. Membership secretary: Marilyn Crompton, #1908-21 Overlea Blvd., Toronto ON M4H 1P2, phone 416-467- 0018 Promenea Chameleon 'Rhys' AM-AOS Plant by Mario and Conni Ferrusi photo PP Executive: President, Yvonne Schreiber, 905-473-3405; Vice- president Laura Liebgott, 905-883-5290; Secretary, Sue Meeting Sunday, Loftus 905-839-8281; Treasurer, John Vermeer, 905-823- September 2, 2516 Other Positions of Responsibility: Program, Mario Ferrusi; Toronto Botanical Plant Doctor, Doug Kennedy; Meeting Set up, Tom Atkinson; Garden, Sales at Vendor and Sales table coordinator, Diane Ryley; Membership, Marilyn Crompton, Eric Terreau, Karen noon, program at 1 Hazelton; Web Master, Max Wilson; Newsletter, Peter and pm. Speaker: Ron Mc Inge Poot; Annual Show, Peter Poot; Refreshments, Joe Hatton, PhD. Ron is currently O’Regan. Conservation Committee, Susan Shaw, Tom the head of administration at Atkinson; Show table, Iryna Bonya. the American Orchid Society. Honorary Life Members: Terry Kennedy, Doug Kennedy, Inge Ron has been an orchid Poot, Peter Poot, Joe O’Regan, Diane Ryley, Wayne Hingston. personality for many many Annual Show: February 16 – 17, 2013 years. He is well known around the world for his many excellent lectures and articles and his tireless dedication to the well being of the orchid world. Ron will be talking to us on Orchid Pests and Diseases, a subject we can all relate to and one that Ron is sure to give a new twist. -

Chapter 4 Phytogeography of Northeast Asia
Chapter 4 Phytogeography of Northeast Asia Hong QIAN 1, Pavel KRESTOV 2, Pei-Yun FU 3, Qing-Li WANG 3, Jong-Suk SONG 4 and Christine CHOURMOUZIS 5 1 Research and Collections Center, Illinois State Museum, 1011 East Ash Street, Springfield, IL 62703, USA, e-mail: [email protected]; 2 Institute of Biology and Soil Science, Russian Academy of Sciences, Vladivostok, 690022, Russia, e-mail: [email protected]; 3 Institute of Applied Ecology, Chinese Academy of Sciences, P.O. Box 417, Shenyang 110015, China; 4 Department of Biological Science, College of Natural Sciences, Andong National University, Andong 760-749, Korea, e-mail: [email protected]; 5 Department of Forest Sciences, University of British Columbia, 3041-2424 mail Mall, Vancouver, B.C., V6T 1Z4, Canada, e-mail: [email protected] Abstract: Northeast Asia as defined in this study includes the Russian Far East, Northeast China, the northern part of the Korean Peninsula, and Hokkaido Island (Japan). We determined the species richness of Northeast Asia at various spatial scales, analyzed the floristic relationships among geographic regions within Northeast Asia, and compared the flora of Northeast Asia with surrounding floras. The flora of Northeast Asia consists of 971 genera and 4953 species of native vascular plants. Based on their worldwide distributions, the 971 gen- era were grouped into fourteen phytogeographic elements. Over 900 species of vascular plants are endemic to Northeast Asia. Northeast Asia shares 39% of its species with eastern Siberia-Mongolia, 24% with Europe, 16.2% with western North America, and 12.4% with eastern North America. -

Nervilia Cumberlegei (Orchidaceae), a Newly Recorded Orchid from Myanmar
Bull. Natl. Mus. Nat. Sci., Ser. B, 47(1), pp. 41–44, February 22, 2021 Nervilia cumberlegei (Orchidaceae), a Newly Recorded Orchid from Myanmar Myo Min Latt1,2, Byung Bae Park1 and Nobuyuki Tanaka3,* 1 Department of Environment and Forest Resources, College of Agriculture and Life Science, Chungnam National University, Republic of Korea 2 Department of Environmental Economic, Policy and Management, University of Forestry and Environmental Sciences, Yezin, Myanmar 3 Department of Botany, National Museum of Nature and Science, 4–1–1 Amakubo, Tsukuba, Ibaraki 305–0005, Japan *E-mail: [email protected] (Received 6 November 2020; accepted 23 December 2020) Abstract Nervilia cumberlegei (Orchidaceae) is recorded in Myanmar for the first time. A description, locality information and photographs are provided. Thus far N. cumberlegei is known only from Taiwan and Thailand. This discovery in Myanmar represents the western edge of the species distribution. Key words: Burma, section Linervia, Tanintharyi, taxonomy, terrestrial orchid. (Pridgeon et al., 2005; Tang et al., 2018) and Introduction flowers followed by a vegetative stem bearing a Nervilia Comm. ex Gaudich. is commonly single photosynthetic leaf (Niissalo et al., 2020). known as ‘shield orchid’. Most species grow in Nervilia plants have antioxidant and antibacterial forested habitats and are shade-demanders (Gale properties (Ruthisha et al., 2018) and are used as et al., 2018). The genus Nervilia is most diverse traditional medicine. Medicinal orchids are con- in tropical Asia, although it is widely distributed siderably threatened by anthropogenic impacts in the Old World tropics, from Australasia and such as habitat destruction, and illegal collection the South-west Pacific Islands to sub-Saharan for commercial and subsistence uses (Pant et al., Africa (Pettersson, 1991; Gale et al., 2015, 2002; Pant, 2013). -
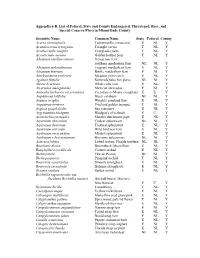
Conservation Appendix 6-B Listed Flora
Appendix 6-B. List of Federal, State and County Endangered, Threatened, Rare, and Special Concern Flora in Miami-Dade County Scientific Name Common Name State Federal County Acacia choriophylla Tamarindillo; cinnecord E NL Y Acanthocereus tetragenus Triangle cactus T NL Y Acoelorraphe wrightii Everglades palm T NL Y Acrostichum aureum Golden leather fern T NL Y Adiantum capillus-veneris Venus hair fern; southern maidenhair fern NL NL Y Adiantum melanoleucum Fragrant maidenhair fern E NL Y Adiantum tenerum Brittle maidenhair fern E NL Y Aeschynomene pratensis Meadow joint-vetch E NL Y Agalinis filifolia Seminole false fox glove NL NL Y Aletris bracteata White colic root E NL Y Alvaradoa amorphoides Mexican alvaradoa E NL Y Amorpha herbacea var.crenulata Crenulate (=Miami) leadplant E E Y Amphitecna latifolia Black calabash NL NL Y Anemia wrightii Wright's pineland fern E NL Y Angadenia berteroi Pineland golden trumpet T NL Y Argusia gnaphalodes Sea rosemary E NL Y Argythamnia blodgettii Blodgett's silverbush E C Y Aristolochia pentandra Marsh's dutchmans pipe E NL Y Asplenium abscissum Cutleaf spleenwort NL NL Y Asplenium dentatum Toothed spleenwort E NL Y Asplenium serratum Wild bird nest fern E NL Y Asplenium verecundum Modest spleenwort E NL Y Asplenium x biscaynianum Biscayne spleenwort NL NL Y Asteraea lobata Lobed croton; Florida treefern NL NL Y Baccharis dioica Broombush falsewillow E NL Y Basiphyllaea corallicola Carter's orchid E NL Y Bletia patula Flor de Pesmo NL NL Y Bletia purpurea Pinepink orchid T NL Y Bourreria cassinifolia Smooth strongback E NL Y Bourreria succulenta Bahama strongback E NL Y Brassia caudata Spider orchid E NL Y Brickellia eupatorioides var. -

CHENOPODIACEAE 藜科 Li Ke Zhu Gelin (朱格麟 Chu Ge-Ling)1; Sergei L
Flora of China 5: 351-414. 2003. CHENOPODIACEAE 藜科 li ke Zhu Gelin (朱格麟 Chu Ge-ling)1; Sergei L. Mosyakin2, Steven E. Clemants3 Herbs annual, subshrubs, or shrubs, rarely perennial herbs or small trees. Stems and branches sometimes jointed (articulate); indumentum of vesicular hairs (furfuraceous or farinose), ramified (dendroid), stellate, rarely of glandular hairs, or plants glabrous. Leaves alternate or opposite, exstipulate, petiolate or sessile; leaf blade flattened, terete, semiterete, or in some species reduced to scales. Flowers monochlamydeous, bisexual or unisexual (plants monoecious or dioecious, rarely polygamous); bracteate or ebracteate. Bractlets (if present) 1 or 2, lanceolate, navicular, or scale-like. Perianth membranous, herbaceous, or succulent, (1–)3–5- parted; segments imbricate, rarely in 2 series, often enlarged and hardened in fruit, or with winged, acicular, or tuberculate appendages abaxially, seldom unmodified (in tribe Atripliceae female flowers without or with poorly developed perianth borne between 2 specialized bracts or at base of a bract). Stamens shorter than or equaling perianth segments and arranged opposite them; filaments subulate or linear, united at base and usually forming a hypogynous disk, sometimes with interstaminal lobes; anthers dorsifixed, incumbent in bud, 2-locular, extrorse, or dehiscent by lateral, longitudinal slits, obtuse or appendaged at apex. Ovary superior, ovoid or globose, of 2–5 carpels, unilocular; ovule 1, campylotropous; style terminal, usually short, with 2(–5) filiform or subulate stigmas, rarely capitate, papillose, or hairy on one side or throughout. Fruit a utricle, rarely a pyxidium (dehiscent capsule); pericarp membranous, leathery, or fleshy, adnate or appressed to seed. Seed horizontal, vertical, or oblique, compressed globose, lenticular, reniform, or obliquely ovoid; testa crustaceous, leathery, membranous, or succulent; embryo annular, semi-annular, or spiral, with narrow cotyledons; endosperm much reduced or absent; perisperm abundant or absent. -

Redalyc.EFFORTS to CONSERVE ENDANGERED TERRESTRIAL
Lankesteriana International Journal on Orchidology ISSN: 1409-3871 [email protected] Universidad de Costa Rica Costa Rica RANGEL-VILLAFRANCO, MONICA; ORTEGA-LARROCEA, M. PILAR EFFORTS TO CONSERVE ENDANGERED TERRESTRIAL ORCHIDS IN SITU AND EX SITU AT TWO NATURAL RESERVES WITHIN CENTRAL MEXICO Lankesteriana International Journal on Orchidology, vol. 7, núm. 1-2, marzo, 2007, pp. 326-333 Universidad de Costa Rica Cartago, Costa Rica Available in: http://www.redalyc.org/articulo.oa?id=44339813068 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative LANKESTERIANA 7(1-2): 326-333. 2007. EFFORTS TO CONSERVE ENDANGERED TERRESTRIAL ORCHIDS IN SITU AND EX SITU AT TWO NATURAL RESERVES WITHIN CENTRAL MEXICO 1 1,2 MONICA RANGEL-VILLAFRANCO & M. PILAR ORTEGA-LARROCEA 1 Departamento de Edafología, Instituto de Geología, Universidad Nacional Autónoma de México. Circuito Exterior de Ciudad Universitaria, México Distrito Federal, 04510. México. 2 Author for correspondence: [email protected] KEY WORDS: in situ conservation, ex situ conservation, orchid fungi isolation, seed banks The natural vegetation in and around Mexico City macrobulon, Epidendrum anisatu, Habenaria strictis- once harbored an unusually high number of plant and sima, Liparis greenwoodiana) (Hágsater et al. 2005). animal (insect) species, including endemics (Vázquez In contrast, in the Chichinautzin Area, eight types 1973, Ceballos & Galindo 1984, Rzedowski 1991). of vegetation can be found. An altitudinal gradient The high diversity in this region has been attributed joint with successive periods of volcanic activity to the unusual topography resulting from a series of are combined and pedogenetic processes through volcanic eruptions that ended ca.