CTRI Trial Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mumbai – 400065
MAHARASHTRA FILM, STAGE & CULTURAL DEV. CORPN. LTD. Dadasaheb Phalke Chitranagari, Goregaon (East), Mumbai – 400065 Details of Empanelled Agencies Name of work:To empanel eligible Agencies to provide security personnel and related services at various shooting locations/ sets of producers in Dadasaheb Phalke Chitranagari. Sr. No Name of the Agency Details of the agency Rates quoted by the Agencies Head Security Security Supervisor Bouncer Lady Fire Head Security Remarks Guard Guard Guard with Guard Marshal Guard Guard gun handling handling charges Charges 1 CISB 302, Centre Point, J.B.Nagar, Andheri Kurla 25097.00 24598.00 26371.00 Nil Nil Nil Nil Nil Nil Nil Road, Andheri East, Mumbai- 400059. Contacts office.. 022-61483333 Name of the contact Person - Cdr Sukhdev Singh. Sr VP. Mobile 9223282586 2 Combat Faciltiy and Shop no. 5, Vastu Labh Building, Jijamata Road, 22304.2021843.20 Nil Nil Nil Nil Nil 2230.42 2184.32 Nil Services Next to Sunita Hospital, Andheri (E) Mumbai – 400093. Name of the person to contact and their designation :- 1. Subhash Darekar (Managing Director) Mobile :-9819846947, 8828544140 2. Mr. Sunil Mishkin 9920769838 3 Eagle SPS India Shop No. 12, Nirmala Co-op Hsg Society Ltd. JP 22843.98 22382.35 24019.06 Nil Nil Nil Nil Nil Nil Nil Road, Andheri (west) Mumbai 400058 Contact number (office) :- 022 26772065/1034 & 022 43594345 Name of person to contact and their designation in the company with mobile number :- 1) Mr. Chitrasen Sharma – Sole Proprietor Mob. No. 9833315799 2) Mr. Mit Chheda Business Head Mob. No. 9768781671 2) Mr. Ritesh Sharma – Operation Head Mob. -

Print Studio
_w§~B© {dÚmnrR> Electoral Roll of Registered Graduate - 2010 ELC. NO. NAME & ADDRESS ELC. NO. NAME & ADDRESS 30001 Form No.: 11011 30002 Form No.: 31512 PAW AR KALPESH PRAKASH PAW AR KALPESH PRAKASH ADD: 1A/8, UNITY COMPLEX RAJAN PADA, P.G. ADD: A-8,UNITY COMPLEX RAJAN PADA,LINK ROAD MARG MALAD (WEST) MUMBAI 400064 MALAD(W) MUMBAI 400064 30003 Form No.: 41140 30004 Form No.: 10861 PAWAR KALPESH RAVINDRA / PAWAR KALYANI SHANKAR ADD: pLOT NO. 254/C-21, PRAGATI CHS LTD ADD: CHANDRA DARSHAN APARTMENT A/5, 1ST GARAI-2,BORIVALI (W) MUMBAI 400091 FLR, HANUMAN NAGAR KATEMANIVALI, KALYAN (E) MUMBAI 30005 Form No.: 26982 30006 Form No.: 37563 / PAWAR KANCHAN RAMESH / PAWAR KANGHAN CHANDOO ADD: R.NO-13, GATE NO-2, ROAD NO-6,R.NO-13, ADD: B-30/002, R.M. ANAND NAGAR, ANAND GATE NO-2, ROAD NO-6,SANTACRUZ NAGAR, DAHISAR (E), MUMBAI MUMBAI 400068 EAST,MUMBAI,400055 30007 Form No.: 8885 30008 Form No.: 10566 PAW AR KAPIL VINAYAK PAWAR KARAN SINGH ADD: ROO M NO.5, KRO PA BLDG., T.H. KATARIA ADD: A-306, SANGAM CO-OP HOU SOC KALINA MARG, BHAGAT LANE MAHIM MUMBAI 400016 MANPADA CST ROAD SANTACRUZ (E) MUMBAI 400098 30009 Form No.: 23512 30010 Form No.: 554 / PAWAR KARUNA DHANAJI / PAWAR KAVITA PRASHANT ADD: 3/1 SATU SADAN BANDREKARWADI ADD: SHREEMUKH SADAN, R.NO 48 SENAPATI JOGESHW ARI [E] MUMBAI 400060 BAPAT X ROAD MAHIM MUMBAI 400016 30011 Form No.: 20129 30012 Form No.: 16064 PAWAR KIRAN HANUMANT / PAWAR KIRAN JANARDAN ADD: 19, DEVGAD NIWAS NARDAS NAGAR, T.P. -

CTRI Trial Data
PDF of Trial CTRI Website URL - http://ctri.nic.in Clinical Trial Details (PDF Generation Date :- Sat, 02 Oct 2021 13:04:58 GMT) CTRI Number CTRI/2012/10/003077 [Registered on: 29/10/2012] - Trial Registered Retrospectively Last Modified On 01/03/2013 Post Graduate Thesis No Type of Trial Interventional Type of Study Drug Preventive Study Design Randomized, Parallel Group, Active Controlled Trial Public Title of Study Comparison of IN-SYB-01 with Levonorgestrel in Women Seeking Emergency Contraception within 120 Hours of Unprotected Intercourse Scientific Title of An Open Label, Phase III Randomized, Parallel Group, Prospective, Multicentre Clinical Trial for Study evaluation of Efficacy and Safety of IN-SYB-O1 in Comparison with Levonorgestrel in Women Seeking Emergency Contraception within 120 Hours of Unprotected Intercourse. Secondary IDs if Any Secondary ID Identifier CT/ IN-SYB-O1/EC/III/11, Version No.: 00; Date: Protocol Number 30/08/2011 Details of Principal Details of Principal Investigator Investigator or overall Name Dr Rane Trial Coordinator (multi-center study) Designation Senior Vice President Affiliation Intas Pharmaceuticals Ltd Address Medical Services Department Intas Pharmaceuticals Ltd 7th floor Premier House 1 Gandhinagar Sarkhej Highway Bodakdev Ahmedabad India Ahmadabad GUJARAT 380054 India Phone 66117401 Fax 26840224 Email [email protected] Details Contact Details Contact Person (Scientific Query) Person (Scientific Name Dr Sonal Mehta Query) Designation Medical Advisor Affiliation Intas Pharmaceuticals Ltd -

Chapter II IKIRQUUCTIQN in This Chapter, Districtwise and Placewise
Chapter I I IKIRQUUCTIQN In this chapter, districtwise and placewise account of the places that the author has visited is recorded. The total number of places recorded is three nundred and two. Gut of these one hundred and fifty five places the author has visited. Other places have been included to maice the information complete. There are 201 places mentioned in various inscrip tions (see Table below) out of which 130 places were visited by the author. In addition 25 places though not mentioned were visited and an account of all such is recorded. Thus this is a Gazetteer-like account of the places which cover the period from 500 A.D. to 1300 A.D. having some antiquarian importance. In giving the account of a place the following points are elaborated. Inscriptional name, geographical, socio logical, and material remains are noted. Places having a|(^ mark were visited by the author. 23 Table I Dynasty Places mentioned Visited in inscriptions Badami Chalukyas 33 20 Rastrakutas 66 41 Kalyani Chalukyas 14 8 Yadavas of fleogiri 70 51 Silahara of N. Konkan 2 2 Silahara of Kolhapur 16 8 Total 201 130 Table 11 a District Information recorded Visited Ahmadnagar 69 19 Dhulia 16 3 Jalgaon 40 15 Kolhapur 28 18 Nasik 28 16 Poona 62 41 Sangall 12 9 Satara 27 12 Sholapur 30 23 Total 302 165 S Places from Ahuiadnagar, Jalgaon, and Satara district con tain Hemadpanti temples and wells, and they have been al ready sui'veyed; so not visited but are recorded. hkm% o t Flactii ia ttMi AhaaUnftgor ulatrlct 1 KftJl«£»oa 18 Kaiaa 35 XakXl 2 Hu;»t>barl 19 -

Indian Bureau of Mines
Registration Under Rule 45 of MCDR 1988 - Indian Bureau of Mines (Ministry of Mines) *********** (Total Records : 1658) For Region: UDAIPUR For ( All ) Business Activity Registration Applying for the period of ( 01/01/2011 to 31/03/2016 ) Sl. Name/ Category/ App. Business Position Address State/ District Contact Details Registration No./ No. Date Activity In Mine Date/ Status 1 M/s Krishna Miners & Mining 3•B, Industrial Estate RAJASTHAN 0294•2491001 (O) IBM/54/2011 Traders Pratapnagar UDAIPUR 0294•2491003 (F) (14/10/2011) (Firm • Registered) Girwa 0294•2425263 (R) 25/08/2011 313003 [email protected] 2 M/s Ashoka Minerals Mining 20 Industrial Estate RAJASTHAN 0294•2491001 (O) IBM/64/2011 (Firm • Registered) Pratapnagar UDAIPUR 0294•2491003 (F) (14/10/2011) 26/08/2011 Girwa 0294•2425263 (R) 313003 [email protected] 3 M/s SHREE DIGVIJAY Mining, End DIGVIJAYGRAM GUJARAT 0288•2344272 (O) IBM/95/2011 CEMENT COMPANY user JAMNAGAR 0288•2344092 (F) (14/10/2011) LIMITED JAMNAGAR (Company) 361140 9099037895 (M) 29/08/2011 [email protected] 4 M/s Bholenath Manganese Trader, Udegarh Mishnawat Kushalgarh RAJASTHAN 079•26891479 (O) IBM/126/2011 Ind. (Firm • Registered) Storage, End Kushalgarh BANSWARA (29/09/2011) 06/09/2011 user 327801 9825225742 (M) [email protected] 5 M/s AJMERA CEMENTS Mining PO. BHANDURI, GUJARAT 02870•281482 (O) IBM/179/2011 PVT. LTD. VERAVAL • JUNAGADH NH JUNAGARH 02870•281483 (F) (14/10/2011) (Company) 8D, MALIYA (HATINA), 10/09/2011 362245 [email protected] 6 M/s Wonder Cement Mining, Wonder Cement Limited RAJASTHAN 01477•307000 (O) IBM/226/2011 Limited Storage, End R. -

Shree Pushkar Chemicals & Fertilisers Limited
PROSPECTUS August 29, 2015 Please read Section 32 of the Companies Act, 2013 Book Building Issue SHREE PUSHKAR CHEMICALS & FERTILISERS LIMITED Our Company was incorporated as ‘Shree Pushkar Petro Products Limited’ a public limited company under the Companies Act, 1956 pursuant to a Certificate of Incorporation dated March 29, 1993 bearing registration number 11-71376 of 1993 and certificate of commencement of business on August 3,1993 issued by the Registrar of Companies, Maharashtra, Mumbai. The name of our Company was changed to ‘Shree Pushkar Chemicals & Fertilisers Limited’ pursuant to fresh certificate of incorporation consequent upon change of name dated March 5, 2012 issued by the Registrar of Companies, Maharashtra, Mumbai. Our corporate identification number is U24100MH1993PLC071376. For further details of our Company, please refer to the chapters titled ‘General Information’ and ‘History and Certain Corporate Matters’ beginning on page numbers 41 and 124, respectively. Registered and Corporate Office: 202, A Wing, Building No. 3, Rahul Mittal Industrial Estate, Sir M.V. Road, Andheri (East), Mumbai – 400 059, Maharashtra Tel. No.: +91 22 4270 2525; Fax No.: +91 22 2850 4242; Company Secretary and Compliance Officer: Kishan Bhargav; Email: [email protected]; Website: www.shreepushkar.com OUR PROMOTERS: PUNIT MAKHARIA AND GAUTAM MAKHARIA PUBLIC ISSUE OF 1,07,69,200 EQUITY SHARES OF FACE VALUE OF ` 10 EACH (“EQUITY SHARES”) OF SHREE PUSHKAR CHEMICALS & FERTILISERS LIMITED (THE “COMPANY” OR THE “ISSUER”) FOR CASH AT A PRICE OF ` 65/- PER EQUITY SHARE (INCLUDING A SHARE PREMIUM OF ` 55/- PER EQUITY SHARE) AGGREGATING UPTO ` 700 MILLION CONSISTING OF A FRESH ISSUE OF 87,42,611 EQUITY SHARES AGGREGATING UPTO ` 568.27 MILLION (THE “FRESH ISSUE”) AND AN OFFER FOR SALE OF UPTO 20,26,589 EQUITY SHARES BY THE SELLING SHAREHOLDER (AS DEFINED IN “DEFINITIONS AND ABBREVIATIONS”) AGGREGATING UP TO ` 131.73 MILLION (THE “OFFER FOR SALE” AND THE “FRESH ISSUE” ARE TOGETHER REFERRED TO AS, THE “ISSUE”). -
Interim Dividend 2020-21
Sr No. First Name Middle Name Last Name Address Pincode Folio Amount 1 AR BHANDARI 49 VIDYUT ABHIYANTA COLONY MALVIYA NAGAR JAIPUR RAJASTHAN 302017 0000IN30001110438445 2,010.00 2 A R PARMAR 3 SAVGUNPARK INDIRANAGAR ROAD OPP WATER TANK NADDAD 387002 00AN1204720011045766 27.00 3 A B FONTES 104 IVTH CROSS KALASIPALYAM NEW EXTENSION BANGALORE 560002 0000000000ANA0026601 37,011.00 4 A SHARI PRASAD NO 15 KANDA SWAMY MUDAWAR ROAD BANGALORE 560005 0000IN30061010285620 34.00 5 A SHYLA SRI NANJUNDESHWARA NILAYA NEAR PATTANAGERE BUS STOP , RAJARAJESWARINAGAR BANGALORE 560039 0000IN30611490415134 302.00 6 A S ROOPA ABM KARNATAKA BANK LTD NO 544 15TH MAIN 50FEET ROAD SRINAGAR BANGALORE 560050 0000IN30214810766094 67.00 7 A RATHNA NO 2646 CHIKKAPETE DODDABALLAPURA BANGALORE 561203 00AN1203320000925976 134.00 8 A SRINIVASA GUPTA 2734 CHANDRA GUPTA ROAD MYSORE 570001 0000000000ANA0042780 1,085.00 9 A SRINIVASA GUPTA 2734 CHANDRA GUPTA ROAD MYSORE KARNATAKA INDIA 570001 0000000000ANA0007274 2,974.00 10 A P MAHENDRANATH S/O LATE PATTABI SETTY ASHWINI NILAYA,13 th CROSS SIT EXTENSION TUMKUR 572103 00AN1203070000138477 268.00 11 A S CHITTARANJAN D NO 589/2 6TH MAIN P J EXTENSION DAVANGERE 577002 0000IN30214810044108 6,507.00 12 A SETHUMADHAVAN NAIR PLOT NO.2020 H 24 B 2 5TH STREET 12TH MAIN ROAD ANNA NAGAR WEST CHENNAI 600040 0000IN30108010395917 168.00 13 A CHINNAPPAN 27/8-B, GROUND FLOOR 3RD MAIN ROAD RAJALAKSHMI NAGAR VELACHERY, CHENNAI 600042 0000IN30232410970115 4.00 14 A PADMANABHAN 5 PONNIE STREET RAJAJI NAGAR VILLIVAKKAM 600049 0000000000ANA0034752 -
List of Eligible Candidates for 6 Weeks Certificate Course in Sports
SPORTS AUTHORITY OF INDIA NETAJI SUBHAS NATIONAL INSTITUTE OF SPORTS:PATIALA Dated : 18-06-2021 JOINING INSTRUCTIONS for Eligible candidates of 6 Weeks Certificate Course-2021 Candidates selected for the admission to Six weeks Certificate Course in Sports Coaching 2021 are advised to read the following instructions carefully and act accordingly: 1. Those who are selected are to register themselves online using link given below Registration will begin on 21-06-2021 and will close by 27-06-2021. https://docs.google.com/forms/d/e/1FAIpQLScSjWEPgIkM3enhH20P9TAFbJLYhP17 tFHuZcLRLUU_JCZaww/viewform?usp=sf_link 2. Course fee is to be deposited online by the candidate using S B Collect From 21-06-2021 to 27-06-2021 as per the details given below: Path of S B Collect: i) https://www.onlinesbi.com ii). Go-SB Collect (By enter top box) iii). New versionselect the check box Proceed iv). State of Institute Select Punjab v). Type of Institute Select Education Institution Go vi). Education Institute name Select Sports Authority of India Submit vii). Select payment category COURSE FEE FOR SIX WEEKS . CERTIFICATE COURSE viii). Fill the form SUBMIT LAST DATE FOR DEPOSITING COURSE FEE IS 27-06-2021 3. If any selected candidate fails to deposit fee and do not register by due date will Not be allowed to join the course. 4. Candidates have to join their respective selected centres for online theory and practical classes. 5. Selected candidates those who have not submitted medical fitness certificate from MBBS/MD Doctor have to submit the fitness certificate stating that he/she is medically fit to undertake physical activities as mentioned in the format enclosed as Annexure-I. -

Kalavara Super Market
Trade Marks Journal No: 1764 , 26/09/2016 Class 35 KALAVARA SUPER MARKET 3190210 22/02/2016 CANARA BANK STAFF CO OPERATIVE SOCIETY LTD trading as ;CANARA BANK STAFF CO OPERATIVE SOCIETY LTD LOGANS ROAD, TELLICHERY - 670 101 SERVICE PROVIDER A COMPANY INCORPORATED UNDER THE INDIAN COMPANIES ACT, 1956 Address for service in India/Agents address: P. C .N. RAGHUPATHY. NEW # 66 (OLD # 38), ADITHANAR SALAI, PUDUPET, CHENNAI - 600 002. Used Since :24/05/2002 To be associated with: 1109970, 1109971 CHENNAI WHOLESALE AND RETAIL SALE OF GROCERIES, FOOD PRODUCTS, COSMETIC AND HOUSEHOLD GOODS. 3501 Trade Marks Journal No: 1764 , 26/09/2016 Class 35 3190318 22/02/2016 SANTOSH PAWAR 3/13 S.M, PATIL SOCIETY NEAR SAI DEEP BUILDING KAJUPADA, BORIVALI EAST, MUMBAI 400063 MAHARASHTRA, INDIA SERVICE PROVIDER AN INDIAN NATIONAL Proposed to be Used MUMBAI BUSINESS MANAGEMENT AND BUSINESS ADMINISTATION BEING SERVICES FALLING IN CLASS-35 3502 Trade Marks Journal No: 1764 , 26/09/2016 Class 35 3190422 19/02/2016 LALLOOJI & SONS SRI RAMESH KUAMR AGARWAL SRI SUSHIL KUMAR AGARWAL SRI JAGDISH KUMAR AGARWAL SRI VINOD KUMAR AGARWAL SRI SUNIL KUMAR AGARWAL SRI VIPUL KUMAR AGARWAL SRI MUKUL KUMAR AGARWAL SRI HIMANSHU AGARWAL SRI NIKHIL AGARWAL SRI UPANSHU AGARWAL SRI DEEPANSHU AGARWAL trading as ;LALLOOJI & SONS A2,SHIVALIK BUSINESS CENTER,OPP. KENSVILLE GOLF ACADEMY,BEHIND RAJPATH CLUB,OFF S G HIGHWAY,AHMEDABAD - 380054,GUJARAT,INDIA. services provider PARTNERSHIP FIRM 3503 Address for service in India/Attorney address: GAURAV SONI, ADVOCATE E-101, SHAKSHAT STUDIO APPARTMENT, NEAR NANDHISWAR MAHADEV MANDIR, MAKARBA ROAD, VEJALPUR - 380051, AHMEDABAD - GUJARAT Used Since :01/10/2010 AHMEDABAD ADVERTISING IN PRINT MEDIA,ADVERTISING IN DIGITAL MEDIA,BUSINESS MANAGEMENT,DAY TO DAY OFFICE FUNCTIONS. -

Print Studio
_w§~B© {dÚmnrR> Electoral Roll of Registered Graduate - 2010 ELC. NO. NAME & ADDRESS ELC. NO. NAME & ADDRESS 38001 Form No.: 32438 38002 Form No.: 7203 / SHETTY V INATHA VIJAY SHETTY VINAY APPU ADD: 17,D-52,AMARWADI NATH MAHADEV ADD: C -205, MAHADEV NAGAR CABIN ROAD LANE,C.P.TANK GIRGAON MUMBAI 400004 BHAYANDER (EAST) DISTRICT THANE 401105 38003 Form No.: 37275 38004 Form No.: 16167 SHETTY VINAY BOJA SHETTY VINAY GANESH ADD: FLAT NO 4, JEEVAN PASAG BLDG, PRABHAT ADD: RMNO.4/5HANUMAN NIWAS BEHIND COLONY, RD NO. 2, OPP BMC OFFICE, SANTACRUZ SHARDA HIGH SCHOOL SAKINAKA MUMBAI (E), MUMBAI 400055 400072 38005 Form No.: 36643 38006 Form No.: 27389 SHETTY VINAY GASNESH / SHETTY VINAYA YADAVA ADD: ROOM NO.4/5, HANUMAN NIVAS BEHIND ADD: 19/1365 ABHYUDAYA NAGAR,19/1365 SHARDA HIGH SCHOOL SAKINAKA MUMBAI ABHYUDAYA 400072 NAGAR,KALACHOWKI,MUMBAI,400033 38007 Form No.: 5644 38008 Form No.: 5726 / SHETTY VINEETA RATNAKAR SHETTY VISHAL MADHUKAR ADD: 58/2617,M.H.B.COLONY GANDHI NAGAR, ADD: ROOM NO.6, ANANT PANTHALE CHAWL BANDRA (EAST), MUMBAI MUMBAI 400051 GOKUL NAGAR, MANVEL PADA ROAD VIRAR (E), THANE 401303 38009 Form No.: 1558 38010 Form No.: 11139 SHETTY VISHWANATH SHEKHAR SHETTY YASHRAJ BHASKAR ADD: 601,FLDRENCE APT OPP VAKOLA CHURCH ADD: A -501, AALAY APTS CHURI WADI GOREGAON NEHRU ROAD SANTA CRUZ (E) MUMBAI 400055 (EAST) MUMBAI 400063 38011 Form No.: 40288 38012 Form No.: 7621 S HETTYA NA VIN SANJIV A S HETY ABHIJIT JA YAW ANT ADD: I/B, DREAMS CHS. LBS MARG FLAT NO. 502 ADD: ROOM NO.1035 NEAR JAYASTAMBH BHA N DUP (W ) 400078 RATNAGIRI 415612 38013 Form No.: 23384 38014 Form No.: 10680 SHETY HARDIK ROHIT / SHETY NEHA MEGHASHAM ADD: 10C-7 EMBEE GEEJAY CO-OP HSG SOC LTD 1st ADD: A /202, DAK SANGHATAN CHS BEHIND FLR NR,GARDEN SAIBABA NAGAR BORIVALI [W] DINDOSHI BUS DEPOT MALAD (E) MUMBAI 400097 MUMBAI 400092 38015 Form No.: 21751 38016 Form No.: 9921 S HETYE AJAY DATTARAM S HETYE AJAY S UDHAKA R ADD: 211/212 -C-4 SARASWATI LOKURAM C OMPLEX ADD: E/102, HANUMAN NAGAR C O-OP. -

Reg. No Name in Full Residential Address Gender Contact No. Email Id REMARK
Reg. No Name in Full Residential Address Gender Contact No. Email id REMARK 55001 BHUREWAR RAJARAM 16/1, HALI (VADGAON Male 9881535351 BABURAO RASTA) HALI, TAL-UDGIR DIST-LATUR 413518 LATUR Maharashtra 55002 MATTA FARHANAAZ 5TH FLOOR, R.NO. 504, R4 Female 9035833990 [email protected] ZUBAIR 75/77 C-BLOCK, BABULA TANK RD NR. J.J. HOSPITAL, MUMBAI 400009 MUMBAI Maharashtra 55003 DEOKATE RUPALI 4725, KUMBHAR VASTI Female 9096314395 DNYANDEO PANHALE VASTI, NIRAWAGAJ, TAL-BARAMATI DIST-PUNE 413102 PUNE Maharashtra 55004 BERAD SEEMA MAHENDRA 137, MATOSHRI NIWAS, NR. Female 9822503675 MIRGANE HOSP. NAGAR MIRGANE HOSP. NAGAR SOLAPUR RD, DAREWADI 414002 AHMEDNAGAR Maharashtra 55005 SARTALE PRANITA PRATIK, CHANDRASEN Female 9595813523 PRAKASH DESHMUKH C/O- SUBHASHCHANDRA RATHOD RH NO-3, RAMTARA HSG SOCY 431001 AURANGABAD Maharashtra 55006 PATIL KAMLESH DHANRAJ FLAT NO.223, SAGAR Female 8983330308 [email protected] DARSHAN E-WING, OPP BANK OF MAHARASHTR PARNAKA, DAHANU(W) 401601 THANE Maharashtra 55007 BADAKH PRATIBHA A/P-KARAJGAON, TAL- Female 02427/232266 9890039159 DNYANDEO NEWASA DIST-AHMEDNAGAR 414105 AHMEDNAGAR Maharashtra 55008 SAPKAL SWATI EKNATH PANCHAL BLDG, H.NO. 31 Female 020/24213871 [email protected] BIBVEWADIGAON, PUNE 37 411037 PUNE Maharashtra 55009 AGRAWAL ROSHANI BAJERIYA SQUARE MASOBA Female 0712/2762577 9579543576 OMPRAKASH LANE, NAGPUR 440018 NAGPUR Maharashtra 55010 YADAV PRIYAMVADA ROOM NO. 12, MANIKA Female 02226878037 9324925023 AMIRCHAND YADAV CHAWL, MULGAON DONGARI, A.K. RD, ANDHERI(E), MUMBAI 400093 MUMBAI -
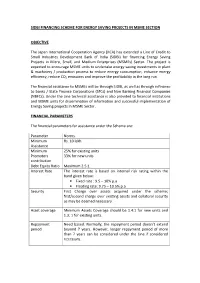
Sidbi Financing Scheme for Energy Saving Projects in Msme Section
SIDBI FINANCING SCHEME FOR ENERGY SAVING PROJECTS IN MSME SECTION OBJECTIVE The Japan International Cooperation Agency (JICA) has extended a Line of Credit to Small Industries Development Bank of India (SIDBI) for financing Energy Saving Projects in Micro, Small, and Medium Enterprises (MSMEs) Sector. The project is expected to encourage MSME units to undertake energy saving investments in plant & machinery / production process to reduce energy consumption, enhance energy efficiency, reduce CO 2 emissions and improve the profitability in the long run. The financial assistance to MSMEs will be through SIDBI, as well as through refinance to banks / State Finance Corporations (SFCs) and Non Banking Financial Companies (NBFCs). Under the Line technical assistance is also provided to financial institutions and MSME units for dissemination of information and successful implementation of Energy Saving projects in MSME Sector. FINANCIAL PARAMETERS The financial parameters for assistance under the Scheme are: Parameter Norms Minimum Rs. 10 lakh Assistance Minimum 25% for existing units Promoters 33% for new units contribution Debt Equity Ratio Maximum 2.5:1 Interest Rate The interest rate is based on internal risk rating within the band given below: • Fixed rate : 9.5 – 10% p.a • Floating rate: 9.75 – 10.5% p.a Security First Charge over assets acquired under the scheme; first/second charge over existing assets and collateral security as may be deemed necessary. Asset coverage Minimum Assets Coverage should be 1.4:1 for new units and 1.3: 1 for existing units. Repayment Need based. Normally, the repayment period doesn’t extend period beyond 7 years. However, longer repayment period of more than 7 years can be considered under the Line if considered necessary.