Analysis of Groundwater Quality in the Vicinity of Kala Sanghian Drain, Jalandhar
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ACADEMIC COUNCIL (For the Term of 01.07.2014 to 30.06.2016)
ACADEMIC COUNCIL (For the term of 01.07.2014 to 30.06.2016) 1. The Vice Chancellor, (Chairman) 14. Dr. A.J.S. Bhanwer, Guru Nanak Dev University, Amritsar. Dean, Faculty of Life Sciences, 2. Director of Public Instructions, Professor, Deptt. of Human Genetics, Chandigarh (Punjab). Guru Nanak Dev University, Amritsar. 3. Dean, Academic Affairs. (Upto 24-08-2015) Guru Nanak Dev University,Amritsar. 15. Dr. Sukhdev Singh, 4. Dean, Students’ Welfare. Dean, Faculty of Physical Education, Guru Nanak Dev University, Amritsar. Professor, Deptt. of Physical Education, 5. Dean, College Development Council. Guru Nanak Dev University, Amritsar. Guru Nanak Dev University, Amritsar. 16. Dr. Sarbjot Singh Behal, 6. Dr. (Mrs.) Jasmeet Sandhu, Dean, Faculty of Physical Planning & Dean, Faculty of Arts & Social Sciences, Architecture, Professor, Deptt. of Sociology, Professor, Deptt. of Architecture, Guru Nanak Dev University, Amritsar. Guru Nanak Dev University, Amritsar. 7. Dr. Sukhdev Singh, 17. Dr. Subodh Kumar, Associate Professor, Deptt. of Agriculture, Dean, Faculty of Sciences, Khalsa College, Amritsar. Professor, Deptt. of Chemistry, Faculty of Agriculture & Forestry, Guru Nanak Dev University, Amritsar. (Upto 14-11-2015) 18. Dr. Shyamal Koley, 8. Dean, Academic Affairs, Dean, Faculty of Sports Medicine & Dean, Faculty of Economics & Business, Physiotherapy, Guru Nanak Dev University, Amritsar. Associate Professor, Deptt. of Sports Medicine (Upto 14-11-2015) and Physiotherapy, 9. Dr. Amit Kauts, Guru Nanak Dev University, Amritsar. Dean, Faculty of Education, 19. Dr. Gurpreet Kaur, Principal, M.G.N. College of Education, Dean, Faculty of Visual Arts & Performing Jalandhar. Arts, 10. Dr. Maninder Lal Singh, Professor, Deptt. of Music, Dean, Faculty of Engineering & Technology, Guru Nanak Dev University, Amritsar. -

Annexure-V State/Circle Wise List of Post Offices Modernised/Upgraded
State/Circle wise list of Post Offices modernised/upgraded for Automatic Teller Machine (ATM) Annexure-V Sl No. State/UT Circle Office Regional Office Divisional Office Name of Operational Post Office ATMs Pin 1 Andhra Pradesh ANDHRA PRADESH VIJAYAWADA PRAKASAM Addanki SO 523201 2 Andhra Pradesh ANDHRA PRADESH KURNOOL KURNOOL Adoni H.O 518301 3 Andhra Pradesh ANDHRA PRADESH VISAKHAPATNAM AMALAPURAM Amalapuram H.O 533201 4 Andhra Pradesh ANDHRA PRADESH KURNOOL ANANTAPUR Anantapur H.O 515001 5 Andhra Pradesh ANDHRA PRADESH Vijayawada Machilipatnam Avanigadda H.O 521121 6 Andhra Pradesh ANDHRA PRADESH VIJAYAWADA TENALI Bapatla H.O 522101 7 Andhra Pradesh ANDHRA PRADESH Vijayawada Bhimavaram Bhimavaram H.O 534201 8 Andhra Pradesh ANDHRA PRADESH VIJAYAWADA VIJAYAWADA Buckinghampet H.O 520002 9 Andhra Pradesh ANDHRA PRADESH KURNOOL TIRUPATI Chandragiri H.O 517101 10 Andhra Pradesh ANDHRA PRADESH Vijayawada Prakasam Chirala H.O 523155 11 Andhra Pradesh ANDHRA PRADESH KURNOOL CHITTOOR Chittoor H.O 517001 12 Andhra Pradesh ANDHRA PRADESH KURNOOL CUDDAPAH Cuddapah H.O 516001 13 Andhra Pradesh ANDHRA PRADESH VISAKHAPATNAM VISAKHAPATNAM Dabagardens S.O 530020 14 Andhra Pradesh ANDHRA PRADESH KURNOOL HINDUPUR Dharmavaram H.O 515671 15 Andhra Pradesh ANDHRA PRADESH VIJAYAWADA ELURU Eluru H.O 534001 16 Andhra Pradesh ANDHRA PRADESH Vijayawada Gudivada Gudivada H.O 521301 17 Andhra Pradesh ANDHRA PRADESH Vijayawada Gudur Gudur H.O 524101 18 Andhra Pradesh ANDHRA PRADESH KURNOOL ANANTAPUR Guntakal H.O 515801 19 Andhra Pradesh ANDHRA PRADESH VIJAYAWADA -

Kala Sanghian, Jaland- Har (Punjab) Kunal Adhikary Chandra Krishi Viswavidyalaya, Mohanpur, Nadia, West Bengal
Annals of Case Reports Adhikary k. Ann Case Rep 2016: G127 Case Report DOI: 10.29011/2574-7754/100027 Integrated Approach of Drain Water Management by User Friend- ly Methods & its Socio- Economic Aspects - Kala Sanghian, Jaland- har (Punjab) Kunal Adhikary Chandra Krishi ViswaVidyalaya, Mohanpur, Nadia, West Bengal *Corresponding author: Kunal Adhikary, Chandra Krishi ViswaVidyalaya, Mohanpur, Nadia, West Bengal, Tel: 741252; E Mail: [email protected] Citation: Adhikary k (2017) Integrated approach of Drain Water Management by user friendly methods & its socio- eco- nomic aspects - Kala Sanghian, Jalandhar (Punjab). Ann Case Rep 2016: G127. DOI: 10.29011/2574-7754/100027 Received Date: 29-11-2016; Accepted Date: 15 December 2016; Published Date: 21 December, 2016 Abstract Water pollution is the major issue in the today’s world and mainly in few states in India, water pollution leads to severe health hazards and loss in yield and crop/soil productivity. Selected site Kala Sanghian drain is located in Jaland- har district which is suffered from rigorous pollution and environmental degradation. Agriculture is the one of the major occupation and bases of the state Punjab, so the irrigation water quality and the first-class water availability for irrigation is also important in context for the crop cultivation. In the proposed project site, the major issue for the stern concern is that, untreated drain water is used for the irrigation purpose by the most of the farmers. Consumption of polluted groundwater has left a large number of people suffering from various diseases, including cancer. Gazipur, Allowal, Badshapur, Mehmuwal Mahla, Kohar Kalan, Athola, Mandala Chana, Gidderpindi, Bahmania, Madala, Isewal and Namajepur villages in Jalandhar district are the worst-hit. -

Village & Townwise Primary Census Abstract, Kapurthala, Part X-A & B, Series-17, Punjab
CENSUS 1971 PARTS X-A & B VILLAGE & TOWN SERIES 17 DIRECTORY PUNJAB VILLAGE & TOWNWISE PRIMARY CENSUS ABSTRACT DISTRICT CENSUS KAPURTHALA HANDBOOK DISTRICT P. L. SONDHI H. S. KWATRA OF THE INDIAN ADMINISTRATIVE SERVICE OF THE PUNJAB CIVil, SERVICE Ex-Officio Director of Census Opemtions Deputy Director of Census Opemtions PUNJAB PUNJAB Motif-- GURDWARA BER SAHIB, SULTANPUR LODHI Gurdwara Be?" Sahib is a renowned place of pilgrimage of the Sikhs. It is situated at Sultanpur Lodhi, 16 miles South of Kapurthala, around a constellation of other Gurdwaras (Sikh Temples) associated with the early life of Guru Nanak Dev. It is n:a,.med after the 'Ber', tree under which Guru Nanak Dev used to meditate. Legend has it that sterile women beget child7'en after takinq leaves of this tree. The old Gu'rdwara was re-constructed by the joint effo'rts of Maharaja Jagatjit Singh of Kapurthala, Maharaja Yadvindra Singh of Patiala and Bhai Arjan Singh of Bagrian. A big fair is held at this Gurdwara on Guru Nanak Dev's birthday. Motif by : J. S. Gill. 15 '40' PUNJAB DISTRICT KAPURTHALA s· KILOIUTRES S o 5 10 15 20 4 8 12 MILES 4 o· 3 " Q TO JUL LlJNDllR <' ~O "'''<, U ""a". I. \.. u .) . 31 DISTRICT 80UNOARV..... POST' TtLEGftAPH OFfiCE "................. P'T TAHSIL BOUNDARY.. _TALlil PRIMARV HEALTH DISTRICT HEADQUARTERS .. CENTRE S IMATERNITY • CHIlD T"HSIL HEADQUARTERS. WELfARE CENTRES ............... - ... $ NATIONAL HIGHWAY .. liECONDARY SCHOOL./COl.LEGE .............•..• , OTHER METAI.LED ROAII.. 45 BROAD GAUGE RAILWAYS WITH STATIOfll. ... RS 4 RIVER .. - CANAL .. UklAII AREA •.. RUT HOUSE .... VILLAQES HAVING POPULATION 5000+ URBAN POPULATION " 50.000 PERSONS 10.000 •.. -

Sno DISTRICT NAME UDISE School Name Area Name Internet Connecti on Type 1 AMRITSAR 3020810602 GSSS GHANUPUR Urban BB 2 AMRITSAR
List of 1500 Schools having Internet Connectivity Internet Area Sno DISTRICT NAME UDISE School_Name Connecti Name on type 1 AMRITSAR 3020810602 GSSS GHANUPUR Urban BB 2 AMRITSAR 03020810801 GHS MAHAL RMSA UPGRADED Urban BB 3 AMRITSAR 3020702202 GHS TANGRA Rural Dongle 4 AMRITSAR 3020606702 GHS WADALA KALAN Rural Dongle 5 AMRITSAR 3020800103 GSSS K.B.D.S. BOYS Urban BB 6 AMRITSAR 3020803002 GSSS PUTLIGHAR Urban BB 7 AMRITSAR 3020802702 GSSS NAWAN KOT G Urban BB 8 AMRITSAR 3020304802 GSSS OTHIAN Rural BB 9 AMRITSAR 3020608202 GSSS KHILCHIAN Rural BB 10 AMRITSAR 3020700402 GSSS DHULKA Rural BB 11 AMRITSAR 3020700202 GSSS KALEKE Rural BB 12 AMRITSAR 3020804503 GSSS GOAL BAGH Urban BB 13 AMRITSAR 3020804602 GHS SHARIFPURA Urban Dongle 14 AMRITSAR 3020500102 GSSS JETHUWAL Rural Dongle 15 AMRITSAR 3020804403 GSSS MALL ROAD G Urban BB 16 AMRITSAR 3020807002 GSSS BAL KALAN Rural BB 17 AMRITSAR 3020804802 GSSS KARAMPURA Urban BB 18 AMRITSAR 3020801003 GHS BHAGTA WALA G Urban Dongle 19 AMRITSAR 3020202402 GSSS LOPOKE Rural BB 20 AMRITSAR 3020900102 GHS ATTARI Rural BB 21 AMRITSAR 3020203302 GSSS SARANGRA Rural Dongle 22 AMRITSAR 3020202102 GHS SAHURA Rural Dongle GHS BABA BAKALA RMSA 23 AMRITSAR 03020601503 Rural BB UPGRADED 24 AMRITSAR 3020600803 GSSS BEAS Rural BB 25 AMRITSAR 3020602503 GSSS BUTALA BOYS Rural BB 26 AMRITSAR 3020604002 GHS DHARDEU Rural BB 27 AMRITSAR 3020602202 GSSS BAL SARAI Rural BB 28 AMRITSAR 3020609601 GSSS MEHTA NANGAL Rural Dongle 29 AMRITSAR 3020605703 GSSS RAYYA BOYS Urban BB 30 AMRITSAR 3020602004 GHS SATHIALA GIRLS -

Complete Merit List for the Post of Mali.Pdf
OFFICE OF DISTRICT & SESSIONS JUDGE, JALANDHAR MERIT LIST OF THE CANDIDATES FOR THE POST OF MALI th th Appl 8 8 Sr. Name of the Qualificatio Categor Standard Standard Additional Rank Sr. Father's Name Address DOB Mobile No. % Age 8th Remarks No. Candidate n y Qualification No. Marks Marks Obtained Total CATEGORY - BC (NO. OF VACANCIES: 01) Abohari Road, Madhav Nagar, H.NO.F- 1 1 181 ASHISH KUMAR ROOP LAL 05-08-1985 8TH BC 9465119576 435 600 72.50 Matric 116, FAZILKA H.NO.2611 A, SEC-24-C, 2 2 453 CHANDER KUMAR BINDA DIN VERMA 13-09-1978 8TH BC 9417171508 468 650 72.00 Matric CHANDIGARH W.NO.13, TEACHER COLONY, SADUL SHAHAR, SRI 3 3 881 VIKRAM KUMAR KRISHAN LAL 17-02-1987 8TH BC 7742866787 403 600 67.17 MA GANGANGA NAGAR, RAJASTHAN VPO TIBBA, BASTI AMARKOT, 4 4 220 GURPREET SINGH HARVINDER SINGH TEH. SULTANPUR LODHI, 14-07-1987 8TH BC 9872750276 390 600 65.00 10+2 KAPURTHALA VILLAGE MANAKHERI, P.O. CASTE 5 5 1164 SHAKEEL MOHD. BUDHU SHAH JHUNER, TEHSIL MALERKOTLA, 06-11-1996 8TH BC 9463522610 687 1060 64.81 CERTIFICATE DISTT. SANGRUR NOT ATTACHED H.NO.571, W.NO.1, GURU 6 6 215 KARAN KUMAR MANGAT RAM NANAK NAGAR, NALSA ROAD, 11-08-1994 8TH BC 9803421069 504 800 63.00 RAJPURA CASTE VILLGE KHUNDI, P.O. KHUNDA, 7 7 1086 YOUNAS KARAM CHAND 22-06-1991 8TH BC 9501462876 601 960 62.60 MATRIC CERTIFICATE DISTT. GURDASPUR NOT ATTACHED R/O VPO SHAMBHU KALAN, TEH. -

SIT List 407.Xlsx
S.No. District Type of SITs Name of the SITs 1 Amritsar GSSS Heir 2 Amritsar GSSS Mall Road (G) Senior Secondary School, Dhardao 3 Amritsar Adarsh Butar, 4 Amritsar GSSS Chawinda Devi 5 Amritsar GSSS Attari (B) 6 Amritsar GSSS Putligarh (G) 7 Amritsar GSSS Mahna Singh Road (G) 8 Amritsar GSSS Town Hall 9 Amritsar GSSS Khilchian 10 Amritsar GSSS Jandiala Guru(B) 11 Amritsar GSSS Gaggomahal. 12 Amritsar GSSS Lopoke V.P. 13 Amritsar GSSS Vichoa 14 Amritsar GSSS Ajnala (B) 15 Amritsar GSSS Jandiala Guru (G) 16 Amritsar GSSS Ball Kalan (B) 17 Amritsar GSSS Bhangali 18 Amritsar GSSS M.S.Gate 19 Amritsar GSSS Majitha 20 Amritsar GSSS Baba Sohan Sing 21 Amritsar GSSS Jethuwal 22 Amritsar GSSS Verka 23 Amritsar GSSS Bohru 24 Amritsar GSSS Chheharta 25 Amritsar GSSS Jabbowall 26 Amritsar GSSS Mehta Nangal 27 Amritsar GSSS Sathiala 28 Amritsar GSSS Trasika 1 29 Barnala GSSS Thikriwala 30 Barnala GSSS Bhadaur (G) 31 Barnala GSSS Pakho Kalan 32 Barnala GSSS Tappa(Boys) 33 Barnala GSSS (B) Dhanaula 34 Bathinda GSSS Chauke 35 Bathinda GSSS Goniana (G) 36 Bathinda GSSS Bathinda (G) 37 Bathinda GSSS Kotha Guru 38 Bathinda GSSS Bhucho Mandi (B) 39 Bathinda GSSS Gobindpura 40 Bathinda GSSS Lehra Mohabat 41 Bathinda Adarsh Sen. Sr. School Nandgarh 42 Bathinda GSSS Mandi Kalan (G) 43 Bathinda GSSS ParaRam Nagar S.No. District Type of SITs Name of the SITs 44 Bathinda GSSS (GSSS) Bhagta Bhai Ka 45 Bathinda GSSS Goniana (B) 46 Bathinda GSSS Mandi Phool 47 Bathinda GSSS Maur Mandi (G) 48 Bathinda GSSS (GSSS) Bhucho Kalan 49 Bathinda GSSS Deshraj Bathinda 50 Bathinda -

List of Promoters Sr
List of Promoters Sr. No. Name of Promoter's & Adress Letter No./Date Valid up to ASK Builders Pvt. Ltd. Sh. Satinder Pal Singh 9-L 1 11574/13.12.2019 12.12.2024 Model Town Jalandhar. Lt. Col (Retd) Sh. Baldev Singh Khinda, Menage 2 House 61 Pir Choudhry road, Kapurthala. 374/11.01.16 10.01.2021 Sh. Parminder Singh S/o Sh. Sant Singh r/o 35 3 558/19.01.2016 18.01.2021 Gopal Park, Kapurthala. Sh. Manpreet Singh S/o Sh. Surinder Singh 214 4 624/19.01.2016 18.02.2021 Guru Gobind Singh Nagar, Jalandhar M/s Glenmore Through Sh. Amarjit S/o Sh. 5 Darshan Lal SCO-4,5 FCI Colony, G.T.B Nagar 2075/17.03.2016 16.03.2021 Jalandhar M/s Pravesh Promoters Pvt. Ltd., Through Sh. Ajay Kumar Kurichh S/o Sh. Kuldip Rai Kurichh, 6 3166/27.04.2016 26.04.2021 SCO 6-7-8, First Floor, Sector 9, Chandigarh M/s Prabhu Niwas Builders Through Sh. Jatinder Singh Khehra r/o House No. 2, Khehra Enclave, 7 6455/11.07.2016 31.12.2021 Rama Mandi, Hoshiarpur Road, Jalandhar Sh. Rajinder Singla S/o Sh. Jagdish Rai Singla 8 M/s Singla Enclave Developers Pvt. Ltd, NB203, 2421/04.04.2016 21.03.2021 Lakshmi Pura, Jalandhar. Sh. Joginder SinghS/o Sh. Gurcharan Singh,r/o 9 7840/05.08.2016 04.08.2021 313-C, New Jawahar Nagar,Jalandhar. Sh. Rupinder Singh Gill, Partner S/o Sh. Lakhbir Singh 10 M/s Gillsons Construction Ltd, Dhak Bhullarai, 9639/07.10.2016 06.10.2021 Hoshiarpur Road, Phagwara M/s Fair Deal Developers Through Sh. -
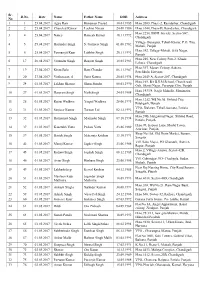
Sr. No D.No. Date Name Father Name DOB Address 1 1 25.04.2017 Agya
Sr. D.No. Date Name Father Name DOB Address No 1 1 25.04.2017 Agya Ram Hanuman Prasad 30.01.1985 H.no.2003, Phase-2, Ramdarbar, Chandigarh 2 2 25.04.2017 Chanchal Kumar Lachmi Narain 24.08.1986 H.no.1040, Phase-II, Ramdarbar, Chandigarh H.no.2250, BSNL Society, Sector-50C, 3 4 25.04.2017 Nancy Ramesh Kumar 10.11.1992 Chandigarh Village- Bansepur, Tehsil-Kharar, P.O. Tira, 4 5 25.04.2017 Harjinder Singh S. Gurmeet Singh 01.08.1982 Mohali, Punjab H.no.342, Village-Mohali, SAS Nagar, 5 6 25.04.2017 Poonamjit Kaur Lakhbir Singh 20.11.1992 Punjab H.no.285, New Colony Part-2, Khuda 6 17 26.04.2017 Verender Singh Ranjeet Singh 10.05.1983 Lahora, Chandigarh H.no.397, Manav Colony, Saketri, 7 19 27.04.2017 Kiran Bala Ram Chander 06.11.1987 Panchkula, Haryana 8 20 27.04.2017 Venkatesan. S Sami Kannu 20.03.1994 H.no.2049 A, Sector-28C, Chandigarh H.no.169, B/s B.S.M School, Church wali 9 24 01.05.2017 Lakhan Sharma Sham Sunder 06.01.1990 Gali, Shanti Nagar, Ferozpur City, Punjab H.no.1433/8, Nagla Mohalla, Manimajra, 10 27 01.05.2017 Harpreet Singh Naib Singh 24.03.1988 Chandigarh H.no.1252, Wd No.05, Sirhind City, 11 28 01.05.2017 Rajan Wadhwa Yaspal Wadhwa 28.06.1993 Fatehgarh, Punjab VPO- Kularan, Tehsil-Samana, Patiala, 12 31 01.05.2017 Sanjeev Kumar Tarsem Lal 02.12.1992 Punjab H.no.39B, Hargobind Nagar, Sirhind Road, 13 32 01.05.2017 Harmanjot Singh Maninder Singh 07.10.1994 Patiala, Punjab H.no.09, Kapoor Lane, Model Town, 14 37 01.05.2017 Kaustubh Vatta Padam Vatta 23.06.1989 Amritsar, Punjab Shop No.165, Old Grain Market, Sunam, 15 39 01.05.2017 Harish Singla Maharaja Krishan 13.10.1991 Sangrur Vill. -

Village & Townwise Primary Census Abstract, Kapurthala, Part XII-A & B
CENSUS OF INDIA 1991 SERIES-20 PUNJAB DISTRICT CENSUS HAND·BOOK PA'RT XII-A & lB VILLAGE & T.OWN DrRECTORY VILLAGE & TOWNWISE .PRIMAR Y CENSUS ABSTRACT DISTRICT KAPURTHALA DIrector of Census Operations Punjab Published by : The Government of Punjab, 1996 PUNJAB DISTRICT KAPURTHALA ~m5 o 5 10 I5 20 Km q.. ~ \. '" q.. ,.. + A.., (J \. q. '" "" () " A.., .. l ;:;p. (" ~• ; ~ \ "z 0 s '{ .. BOUNDARY, DISTRICT _.-..- C.O. BLOCKS TAHSIL A NAOALA " C.D. BLOCK .' .. ........ .. , .. ...... " .. 1"" "e B I(APURTHALA H€ADOUARTERS: DlSTRlCTj TAHSIL .. ... .. .. @; @ C SUL TANPUR LOOM' NATIONAL HIGHWAY .......... .... .•. •. .. _...;,;N""'H.;..'_ o PHAGWARA IMPORTANT METALLED ROAD .. ........ .. " .... e' _-=__ RA!lWAY LINE WITH STATION, BROAD GAUGE: eo •• ' _~ RIVER AND STREAM .. .. .. .. .' .. .. ~ VILLAGE HAVING 5000 AND A90JE POPULATION WITH NAME ... ...... .. ...... .. .. .. .. .. .... Oh«wan• All Ixlondaries 0,. up«lted ullo I~ D«:embor, 1989. URBAN AREA WITH POP\JLATION SIZE CLASS II S IV •• POST AND TELEGRAPH OFFICE .. .. .. .. .. .. .. .. .. PTO DEGREE COLl.EG£ AND TECt-tlICAL INSTITUTION.. .. I!!!l m REST HOUSE RH DISTRICT H£ADQUARTERS IS AlSO TAHSL HEADQUARTERS 80sed upon Survey 01 IIIdkl map .llh the permission 01 the Sur~eyor 0._11 .f 1MiI. © GovlI'nmIIII of In4Ia Cop,,~t. 897. CENSUS OF INDlA-199i A-CENTRAL GOVERNMENT PUBLICATIONS ··Tho publications relating to Punjab bear Series No. 20 and will bo published as follows :- part I·A Administration Report- Enumeration (for official use only). Pa.rt l-B Admi nistration Report-Ta bulatio n (for official use only), "- Part II·A General Population Tables 1 and and r Combined Volume. Pan lI·B Primary Census Abstract J Part III General Economic Tables. -

Sr No Roll No Name Father Name Date of Birth Sex Category School
Sr No Roll No Name Father Name Date of birth Sex Category School Name District MAT SAT GTOBT 1 329120320141 JASPREET KAUR SWARANJEET SINGH 6/2/1999 2 2 GSSS SEHNA BARNALA 72 49 121 2 329120322794 DILRAJDEEP SINGH KEWALJEET SINGH 19/2/2000 1 1 GHS NAINEWAL BARNALA 71 46 117 3 329120322793 GURPREET SINGH HARDEV SINGH 6/7/1999 1 1 GHS NAINEWAL BARNALA 61 52 113 4 329120322789 RAMANDEEP KAUR GURMEET SINGH 23/10/1998 2 1 GHS NAINEWAL BARNALA 66 45 111 5 329120321005 GAGANDEEP KAUR GURDEEP SINGH 18/10/1999 2 3 GSSS SUKHPUR BARNALA 68 42 110 6 329120321012 PARDEEP SINGH JAGSEER SINGH 22/1/2000 1 3 GHS ASPAL KHURD P.O. KOTDUNA BARNALA 63 46 109 7 329120321061 MANJOTKAUR KARMJEET SINGH 30/6/1999 2 3 GMS KHIALI BARNALA 62 45 107 8 329120222527 SIMRANJIT KAUR HARWINDER SINGH 20/3/2000 2 1 GGSSS BHADAUR BARNALA 66 39 105 9 329120322536 RAMNDEEP KAUR MENGAL SINGH 17/6/1999 2 1 GHS BHADALWAD BARNALA 62 43 105 10 329120322709 SUKHPREET KAURT AVTAR SINGH 17/7/2000 2 1 SDMGGSSS TAPA BARNALA 56 49 105 11 329120322797 PARAMRAJ SHARMA NOHAR CHAND 27/10/2000 1 1 GSSS TAPA BARNALA 66 39 105 12 329120321043 AMANDEEP SINGH SUKHWINDER SINGH 20/6/1999 1 3 GHS NAIWALA BARNALA 65 39 104 13 329120322773 NEHA MURTAJA ALI 6/8/1999 2 1 GHS HANDAYIYA BARNALA 63 40 103 14 329120222509 SHUBHDEEP KAUR MALKIT SINGH 6/11/2000 2 1 GHS KHUDDI KHURD BARNALA 66 36 102 15 329120322845 HARMANDEEEP KAUR HARJEET SINGH 29/12/1999 2 1 GSSS SEHNA BARNALA 66 36 102 16 329120320197 RAMANDEEP KAUR BALDEV SINGH 15/12/1998 2 2 GGSSS BHADAUR BARNALA 63 39 102 17 329120322622 KARAMJEET SINGH MAJOR SINGH 26/11/1999 1 1 GHS DARAJ TEH. -
S. No. District School Subject Vacancy 1 Amritsar GGHS NANGAL MEHTA
District School Subject Vacancy S. No. 1 Amritsar GGHS NANGAL MEHTA Punjabi 1 2 Amritsar GGHS SATHIALA Punjabi 1 3 Amritsar GGS ATTARI Punjabi 1 4 Amritsar GGSS AJNALA Punjabi 1 5 Amritsar GGSS BUTALA Punjabi 1 6 Amritsar GGSS TAPIALA Punjabi 3 7 Amritsar GHS BHALA PIND Punjabi 1 8 Amritsar GHS BHILOWAL PACCA Punjabi 2 9 Amritsar GHS BHINDI AULAKH Punjabi 2 10 Amritsar GHS BUDDA THEH Punjabi 1 11 Amritsar GHS BUTTAR DHARDEO Punjabi 1 12 Amritsar GHS DAUKE Punjabi 1 13 Amritsar GHS DHARIWAL KALER Punjabi 1 14 Amritsar GHS GAGGAR BHANA Punjabi 1 15 Amritsar GHS JALALPURA Punjabi 1 16 Amritsar GHS JASRAUR Punjabi 3 17 Amritsar GHS JATTA PACHHIAN Punjabi 1 18 Amritsar GHS JHANDER Punjabi 1 19 Amritsar GHS KAKKAR Punjabi 1 20 Amritsar GHS MEHARBANPURA Punjabi 2 21 Amritsar GHS PREETNAGAR Punjabi 1 22 Amritsar GHS RASOOLPUR KALAN Punjabi 1 23 Amritsar GHS SUDHAR RAJPUTAN Punjabi 1 24 Amritsar GHS UGGAR AULAKH Punjabi 1 25 Amritsar GHS WADALA KALAN WADALA KHURD Punjabi 1 26 Amritsar GHS WARRAICH Punjabi 1 27 Amritsar GMS ARJUN MANGA Punjabi 1 28 Amritsar GMS AWAN Punjabi 1 29 Amritsar GMS BHANGWAN Punjabi 1 30 Amritsar GMS BHATTIKE Punjabi 1 31 Amritsar GMS BHILOWA RMSA UPGRADED Punjabi 1 32 Amritsar GMS CHAINPUR BALLAGGAN Punjabi 1 33 Amritsar GMS CHOWNK MEHTA Punjabi 1 34 Amritsar GMS DAGG Punjabi 1 35 Amritsar GMS DAUKE UPGRADED Punjabi 1 36 Amritsar GMS DHARIWAL KALER RMSA UPGRADED Punjabi 1 37 Amritsar GMS DUJJOWAL Punjabi 1 38 Amritsar GMS JAFARKOT Punjabi 1 39 Amritsar GMS JAMALPUR Punjabi 1 40 Amritsar GMS JATHAUL Punjabi 1 41 Amritsar