Drinking Water Quality and Environmental Monitoring in Rural Areas of District Malir Karachi
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Population Distribution in Sindh According to Census 2017 (Population of Karachi: Reality Vs Expectation)
Volume 3, Issue 2, February – 2018 International Journal of Innovative Science and Research Technology ISSN No:-2456 –2165 Population Distribution in Sindh According to Census 2017 (Population of Karachi: Reality vs Expectation) Dr. Faiza Mazhar TTS Assistant Professor Geography Department. Government College University Faisalabad, Pakistan Abstract—Sindh is our second largest populated province. Historical Populations Growth of Sindh It has a great role in culture and economy of Pakistan. Karachi the largest city of Pakistan in terms of population Census Year Total Population Urban Population also has a unique impact in development of Pakistan. Now 1951 6,047,748 29.23% according to the current census of 2017 Sindh is again 1961 8,367,065 37.85% standing on second position. Karachi is still on top of the list in Pakistan’s ten most populated cities. Population of 1972 14,155,909 40.44% Karachi has not grown on an expected rate. But it was due 1981 19,028,666 43.31% to many reasons like bad law and order situation, miss management of the Karachi and use of contraceptive 1998 29,991,161 48.75% measures. It would be wrong if it is said that the whole 2017 47,886,051 52.02% census were not conducted in a transparent manner. Source: [2] WWW.EN.WIKIPEDIA.ORG. Keywords—Component; Formatting; Style; Styling; Insert Table 1: Temporal Population Growth of Sindh (Key Words) I. INTRODUCTION According to the latest census of 2017 the total number of population in Sindh is 48.9 million. It is the second most populated province of Pakistan. -

UNIVERSITA CA'foscari VENEZIA CHAUKHANDI TOMBS a Peculiar
UNIVERSITA CA’FOSCARI VENEZIA Dottorato di Ricerca in Lingue Culture e Societa` indirizzo Studi Orientali, XXII ciclo (A.A. 2006/2007 – A. A. 2009/2010) CHAUKHANDI TOMBS A Peculiar Funerary Memorial Architecture in Sindh and Baluchistan (Pakistan) TESI DI DOTTORATO DI ABDUL JABBAR KHAN numero di matricola 955338 Coordinatore del Dottorato Tutore del Dottorando Ch.mo Prof. Rosella Mamoli Zorzi Ch.mo Prof. Gian Giuseppe Filippi i Chaukhandi Tombs at Karachi National highway (Seventeenth Century). ii AKNOWLEDEGEMENTS During my research many individuals helped me. First of all I would like to offer my gratitude to my academic supervisor Professor Gian Giuseppe Filippi, Professor Ordinario at Department of Eurasian Studies, Universita` Ca`Foscari Venezia, for this Study. I have profited greatly from his constructive guidance, advice, enormous support and encouragements to complete this dissertation. I also would like to thank and offer my gratitude to Mr. Shaikh Khurshid Hasan, former Director General of Archaeology - Government of Pakistan for his valuable suggestions, providing me his original photographs of Chuakhandi tombs and above all his availability despite of his health issues during my visits to Pakistan. I am also grateful to Prof. Ansar Zahid Khan, editor Journal of Pakistan Historical Society and Dr. Muhammad Reza Kazmi , editorial consultant at OUP Karachi for sharing their expertise with me and giving valuable suggestions during this study. The writing of this dissertation would not be possible without the assistance and courage I have received from my family and friends, but above all, prayers of my mother and the loving memory of my father Late Abdul Aziz Khan who always has been a source of inspiration for me, the patience and cooperation from my wife and the beautiful smile of my two year old daughter which has given me a lot courage. -

Aaaaaaaaaaaaa Type of Branch S No Branch Code Cluster
Sameday Centralized and Decentralised branches for Local Centralized and Decentralised branches for Intercity Branch Type of NIFT / NON- S No Cluster District Region Name Of Branch Clearing Clearing Clearing Code Branch NIFT AREA Branches Inward Outward Inward Outward a a a a a a a a a a a a a 1 0387 NORTH HARIPUR DISTRICT ISLAMABAD RETAIL MAIN BAZAR BRANCH NIFT AREA NO Centralized (CPU - ISLAMABAD) Centralized (CPU - ISLAMABAD) Centralized (CPU - ISLAMABAD) Centralized (CPU - ISLAMABAD) 2 0465 NORTH HARIPUR DISTRICT ISLAMABAD RETAIL VILLAGE HATTAR NIFT AREA NO Centralized (CPU - ISLAMABAD) Centralized (CPU - ISLAMABAD) Centralized (CPU - ISLAMABAD) Centralized (CPU - ISLAMABAD) 3 0252 NORTH ABBOTTABAD DISTRICT ISLAMABAD RETAIL PINE VIEW ROAD NIFT AREA NO Centralized (CPU - ISLAMABAD) Centralized (CPU - ISLAMABAD) Centralized (CPU - ISLAMABAD) Centralized (CPU - ISLAMABAD) 4 0235 NORTH HARIPUR DISTRICT ISLAMABAD RETAIL AKBAR PLAZA (SABZI NIFT AREA NO Centralized (CPU - ISLAMABAD) Centralized (CPU - ISLAMABAD) Centralized (CPU - ISLAMABAD) Centralized (CPU - ISLAMABAD) 5 0571 NORTH HARIPUR DISTRICT ISLAMABAD RETAIL HAVELIAN NIFT AREA NO Centralized (CPU - ISLAMABAD) Centralized (CPU - ISLAMABAD) Centralized (CPU - ISLAMABAD) Centralized (CPU - ISLAMABAD) 6 0990 NORTH ABBOTTABAD DISTRICT ISLAMABAD RETAIL MANSEHRA NIFT AREA NO Centralized (CPU - ISLAMABAD) Centralized (CPU - ISLAMABAD) Centralized (CPU - ISLAMABAD) Centralized (CPU - ISLAMABAD) 7 0203 NORTH HARIPUR DISTRICT ISLAMABAD RETAIL KHALABAT TOWNSHIP NIFT AREA NO Centralized (CPU -

SAARC Workshop on Drought Risk Management in South Asia
Organised by SAARC Disaster Management Centre, New Delhi In collaboration with Afghanistan National Disaster Management Authority SAARC Workshop on Landslide Risk Management in South Asia | 177 | SAARC Workshop on Landslide Risk Management in South Asia Contents Messages (v) Drought Risk Management in South Asia 1 SAARC Disaster Management Centre, New Delhi Drought Impacts and Potential for their Mitigation in Southern and Western Afghanistan 27 Kamal Bhattacharyya, Pir Mohammad Azizi, Sayed Sharif Shobair and Mohammad Yasin Mohsini Drought and Human Suffering in Afghanistan 44 Abdul Khabir ALIM and Sayed Sharif SHOBAIR Drought Management Scenarios in Bangladesh 50 Dr. Abu Wali Raghib Hassan and Md. Mezbanur Rahman Current Status on Drought Risk Management in India 63 Atanu Purkayastha & P.J. Soundararajan Monitoring and Early Warning of Drought in India 78 Jayanta Sarkar Agricultural Drought Management in India 88 B. Venkateswarlu Rainfall Characteristics and its Distribution in Maldives 105 Ahmed Rasheed Drought Risk Management in Nepal 111 Hem Raj Regmi Drought Mitigation in Pakistan: Current Status and Options for Future Strategies 120 Shahid Ahmad, Zahid Hussain, Asaf Sarwar Qureshi, Rashida Majeed and Mohammad Saleem Drought Management in Sri Lanka 164 Lalani Imbulana | 179 | 8 August 2010 Historically disaster management in South Asia began with management of droughts. Successive droughts and the resultant famines that killed millions impelled the colonial administration to develop famine codes that became the basis of relief manuals in post independent era. Large scale improvement of agriculture and irrigation in many areas and diversification of economy in the recent decades have blunted the impact of drought at the macro level, but periodic drought still remains the most creeping and devastating disaster which affects millions of rural communities of South Asia. -

Investigation of Mycobiota from Bovine Milk Samples Vend in Malir District, Karachi
PERWEEN ET AL (2014), FUUAST J. BIOL., 4(2): 229-232 INVESTIGATION OF MYCOBIOTA FROM BOVINE MILK SAMPLES VEND IN MALIR DISTRICT, KARACHI RUBINA PERWEEN*1, KANWAL NAZIM2, FARAH KISHWAR1, ANILA ANWAR1 AND S. SHAHID SHAUKAT3 1Department of Chemistry, Federal Urdu University of Arts, Science and Technology, Karachi, Pakistan 2Marine Reference Collection and Resource Center, University of Karachi, Karachi, Pakistan 3Institute of Environmental Studies, University of Karachi, Karachi, Pakistan *Corresponding author e-mail: r_ [email protected] Abstract Fungi are spoilage organisms in food microbiology since some molds produce mycotoxins and aflatoxin that can cause some severe health concern in humans. In this perspective the present study was designed to determine the mycobiota in fresh bovine milk samples available for residents of Malir District, Karachi, employing culture plate technique. For this task Potato dextrose agar (PDA) and Sabouraud dextrose agar were synthesized for the purpose of the recovery of fungi from the samples. There were seven genera of fungi Alterneria, Aspergillus, Fussarium, Mucor, Penicillium, Pythium and Rhizopus was isolated from this District of Karachi while, A. flavus was recovered as most abundant fungus. Introduction Fungi are considered spoilage organisms in food microbiology. When conditions are not favorable for the growth of bacteria, yeasts and molds will take over and spoil the food items. Fungi secrete digestive enzymes in order to break down complex food sources they can absorb (Srivastava, 2008). The real food-poisoning concern of mold is the possible production of mycotoxins and aflatoxin by some molds which cause hepatic cancer in humans (Hui, 1992; Johny et al., 2012). -

List of Dehs in Sindh
List of Dehs in Sindh S.No District Taluka Deh's 1 Badin Badin 1 Abri 2 Badin Badin 2 Achh 3 Badin Badin 3 Achhro 4 Badin Badin 4 Akro 5 Badin Badin 5 Aminariro 6 Badin Badin 6 Andhalo 7 Badin Badin 7 Angri 8 Badin Badin 8 Babralo-under sea 9 Badin Badin 9 Badin 10 Badin Badin 10 Baghar 11 Badin Badin 11 Bagreji 12 Badin Badin 12 Bakho Khudi 13 Badin Badin 13 Bandho 14 Badin Badin 14 Bano 15 Badin Badin 15 Behdmi 16 Badin Badin 16 Bhambhki 17 Badin Badin 17 Bhaneri 18 Badin Badin 18 Bidhadi 19 Badin Badin 19 Bijoriro 20 Badin Badin 20 Bokhi 21 Badin Badin 21 Booharki 22 Badin Badin 22 Borandi 23 Badin Badin 23 Buxa 24 Badin Badin 24 Chandhadi 25 Badin Badin 25 Chanesri 26 Badin Badin 26 Charo 27 Badin Badin 27 Cheerandi 28 Badin Badin 28 Chhel 29 Badin Badin 29 Chobandi 30 Badin Badin 30 Chorhadi 31 Badin Badin 31 Chorhalo 32 Badin Badin 32 Daleji 33 Badin Badin 33 Dandhi 34 Badin Badin 34 Daphri 35 Badin Badin 35 Dasti 36 Badin Badin 36 Dhandh 37 Badin Badin 37 Dharan 38 Badin Badin 38 Dheenghar 39 Badin Badin 39 Doonghadi 40 Badin Badin 40 Gabarlo 41 Badin Badin 41 Gad 42 Badin Badin 42 Gagro 43 Badin Badin 43 Ghurbi Page 1 of 142 List of Dehs in Sindh S.No District Taluka Deh's 44 Badin Badin 44 Githo 45 Badin Badin 45 Gujjo 46 Badin Badin 46 Gurho 47 Badin Badin 47 Jakhralo 48 Badin Badin 48 Jakhri 49 Badin Badin 49 janath 50 Badin Badin 50 Janjhli 51 Badin Badin 51 Janki 52 Badin Badin 52 Jhagri 53 Badin Badin 53 Jhalar 54 Badin Badin 54 Jhol khasi 55 Badin Badin 55 Jhurkandi 56 Badin Badin 56 Kadhan 57 Badin Badin 57 Kadi kazia -

Rapid Need Assessment Report Monsoon Rains Karachi Division Th Th 24 – 27 August 2020
Rapid Need Assessment Report Monsoon Rains Karachi Division th th 24 – 27 August 2020 Prepared by: Health And Nutrition Development Society (HANDS) Address: Plot #158, Off M9 (Karachi – Hyderabad) Motorway, Gadap Road, Karachi, Pakistan Web: www.hands.org.pk Email: [email protected] Ph: (0092-21) 32120400-9 , +92-3461117771 1 | P a g e Table of Contents 1. Introduction .................................................................................................................... 3 1.1. Background............................................................................................................. 3 1.2. Objectives ............................................................................................................... 3 2. Methodology .................................................................................................................. 4 Situation at Model Town after Heavy Rains ....................................................................... 4 Situation at Model Town after Heavy Rains ....................................................................... 4 3. Findings ......................................................................................................................... 5 3.1. District East............................................................................................................. 6 3.2. Major disaterous events in East district ................................................................... 6 3.3. District Malir ........................................................................................................... -

2013-2014 Development Budget
1 SC22051(051) DEVELOPMENT (REVENUE) Rs Charged: ______________ Voted: 33,098,087,000 ______________ Total: 33,098,087,000 ______________ ____________________________________________________________________________________________ AGRICULTURE DEPARTMENT ____________________________________________________________________________________________ AGRICULTURE RESEARCH ____________________________________________________________________________________________ P./ADP DDO Functional-Cum-Object Classification & Budget NO. NO. Particular Of Scheme Estimates 2013 - 2014 ____________________________________________________________________________________________ Rs 04 ECONOMIC AFFAIRS 042 AGRI,FOOD,IRRIGATION,FORESTRY & FISHING 0421 AGRICULTURE 042103 AGRICULTURAL RESEARCH & EXTENSION SERVIC HD9337 DIRECTOR GENERAL AGRICULTURE RESEARCH SINDH. ADP No : 0001 HD09100137 Development and Promotion of Quality Seed through Public 134,896,000 Private Partnership in Sindh A03970 Others 134,896,000 TA9384 DY.DIRECTOR RICE RESEARCH STATION THATTA ADP No : 0002 TA12131410 Rehabilitation of Rice & Cotton Research Station Thatta 34,826,000 A03970 Others 34,826,000 KR9613 SECRETARY AGRICULTURE ADP No : 0003 HD12138010 Establishment of Agriculture Services Complex and Advisory 10,520,000 Centers in Sindh A03970 Others 10,520,000 HD9337 DIRECTOR GENERAL AGRICULTURE RESEARCH SINDH. ADP No : 0004 KA11120007 Development of Bio - Pesticide for mangement of vegetable 9,414,000 pests. A03970 Others 9,414,000 LA9404 DIRECTOR QUAID-E-AWAM ARI LARKANA ADP No : 0005 LA11120008 -
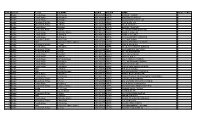
S NO. District Tehsil UC NAME SEMIS PREFIX NAME NO of STAFF 1
S NO. District Tehsil UC NAME SEMIS PREFIX NAME NO OF STAFF 1 Badin Tando Bago 1-Chabralo 401040676 GBPS KANDO FAQEER 1 2 Badin Tando Bago 8-Khadhro 401040043 GBPS HAJI ZAKIR KHOSO 2 3 Badin Tando Bago 6-Pangrio 401040084 GBPS JAMIA HANIFA SULTAN 1 4 Badin Golarchi-S.F.Rao 6-Gharo 401020165 GBPS SADDIQUE JAT 1 5 Badin Golarchi-S.F.Rao 6-Gharo 401020405 GBPS GHULAM SHAH 1 6 Badin Tando Bago 9-Dadah 401040131 GGPS BAKHO CHANDIO 1 7 Badin Golarchi-S.F.Rao 4-Tarai 401020424 GBPS ALI MUHAMMAD NOTKANI 1 8 Badin Tando Bago 3-Khalifo Qasim 401040242 GBPS AHMED ODHEJO 1 9 Badin Tando Bago 6-Pangrio 401040672 GBPS AMIR BUX LUND 1 10 Badin Tando Bago 6-Pangrio 401040223 GBPS HAJI GANHWER AHMDANI 1 11 Badin Golarchi-S.F.Rao 1-Khorwah 401020329 GBPS GUL MIR SHAH 1 12 Badin Matli 6-Ghulam Shah Laghari 401030169 GGPS JAN MUHAMMAD LAGHARI 1 13 Badin Golarchi-S.F.Rao 4-Tarai 401020357 GBPS SHADI NOTKANI 1 14 Badin Tando Bago 8-Khadhro 401040581 GBPS GHULAM HUSSAIN GURGAZ 1 15 Badin Golarchi-S.F.Rao 4-Tarai 401020526 GBPS HAJI LEEMON NOTKANI 1 16 Badin Golarchi-S.F.Rao 4-Tarai 401020419 GBPS DIL MURAD BROHI 1 17 Badin Tando Bago 9-Dadah 401040163 GGPS SULTAN SENHRO 1 18 Badin Tando Bago 4-Khoski 401040077 GBPS SOBHO KOLHI 1 19 Badin Tando Bago 10-Tando Bago 401040056 GBPS ALLAH DINO CHANDIO 1 20 Badin Tando Bago 8-Khadhro 401040537 GBPS GUL MUHAMMAD SHORO 1 21 Badin Tando Bago 9-Dadah 401040614 GBPS SACHAL MALLAH 1 22 Badin Tando Bago 8-Khadhro 401040547 GBPS KHAIR MUHAMMAD KHOSO 1 23 Badin Matli 3-Malhan 401030570 GBPS PIR BUX KHOSO 1 24 Badin Matli 12-Haji -
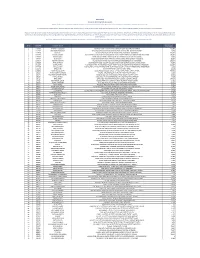
Sr.No ACCOUNT Customer Name Address Balance As of 03/12/2020
Public Notice Auction of Gold Ornaments & Valuables Finance facilities were extended by JS Bank Limited to its customers mentioned below against the security of deposit and pledge of Gold ornaments/valuables. The customers have neglected and failed to repay the finances extended to them by JS Bank Limited along with the mark-up thereon. The current outstanding liability of such customers is mentioned below. Notice is hereby given to the under mentioned customers that if payment of the entire outstanding amount of finance along with mark-up is not made by them to JS Bank Limited within 15 days of the publication of this notice, JS Bank Limited shall auction the Gold ornaments/valuables after issuing public notice regarding the date and time of the public auction and the proceeds realized form such auction shall be applied towards the outstanding amount due and payable by the customers to JS Bank Limited. No further public notice shall be issued to call upon the customers to make payment of the outstanding amounts due and payable to JS Bank Limited as mentioned hereunder: Balance as of Sr.No ACCOUNT Customer Name Address 03/12/2020 1 1148605 NAVEED ALI SOOMRO HOUSE NO 7 489 A SHE EDKI KHOHI SIDIQUE MORI SHIKAR PUR SHIKARPUR 268,787 2 1312357 MOHAMMAD MANSHA VPO KHAS KUTYALA SHA IKAIN BACHER MANDI BAHA DU DIN MANDI BAHAUDDIN 13,938 3 1259818 RAJAB ALI QUAID EAZAM ROAD HAB IB CHOWK NEAR JACOBABAD JACOBABAD 753,623 4 1475296 WAQAR HUSSAIN JAJJA AUR PO KHAS KO T SHER MUHAMMAD TEH PHALIA DIS TT MANDI BAHAUDDIN MANDI BAHAUDDIN 181,820 5 1184967 -
Province Wise Provisional Results of Census - 2017
PROVINCE WISE PROVISIONAL RESULTS OF CENSUS - 2017 ADMINISTRATIVE UNITS POPULATION 2017 POPULATION 1998 PAKISTAN 207,774,520 132,352,279 KHYBER PAKHTUNKHWA 30,523,371 17,743,645 FATA 5,001,676 3,176,331 PUNJAB 110,012,442 73,621,290 SINDH 47,886,051 30,439,893 BALOCHISTAN 12,344,408 6,565,885 ISLAMABAD 2,006,572 805,235 Note:- 1. Total Population includes all persons residing in the country including Afghans & other Aliens residing with the local population 2. Population does not include Afghan Refugees living in Refugee villages 1 PROVISIONAL CENSUS RESULTS -2017 KHYBER PAKHTUNKHWA District Tehsil POPULATION POPULATION ADMN. UNITS / AREA Sr.No Sr.No 2017 1998 KHYBER PAKHTUNKHWA 30,523,371 17,743,645 MALAKAND DIVISION 7,514,694 4,262,700 1 CHITRAL DISTRICT 447,362 318,689 1 Chitral Tehsil 278,122 184,874 2 Mastuj Tehsil 169,240 133,815 2 UPPER DIR DISTRICT 946,421 514,451 3 Dir Tehsil 439,577 235,324 4 *Shringal Tehsil 185,037 104,058 5 Wari Tehsil 321,807 175,069 3 LOWER DIR DISTRICT 1,435,917 779,056 6 Temergara Tehsil 520,738 290,849 7 *Adenzai Tehsil 317,504 168,830 8 *Lal Qilla Tehsil 219,067 129,305 9 *Samarbagh (Barwa) Tehsil 378,608 190,072 4 BUNER DISTRICT 897,319 506,048 10 Daggar/Buner Tehsil 355,692 197,120 11 *Gagra Tehsil 270,467 151,877 12 *Khado Khel Tehsil 118,185 69,812 13 *Mandanr Tehsil 152,975 87,239 5 SWAT DISTRICT 2,309,570 1,257,602 14 *Babuzai Tehsil (Swat) 599,040 321,995 15 *Bari Kot Tehsil 184,000 99,975 16 *Kabal Tehsil 420,374 244,142 17 Matta Tehsil 465,996 251,368 18 *Khawaza Khela Tehsil 265,571 141,193 -
Summary (Lots : 1 & 2) Lot # 2 Supply and Delivery Of
SUMMARY (LOTS : 1 & 2) SUPPLY AND DELIVERY OF SCIENCE LAB MATERIAL LOT # 1 Karachi, Hyderabad, and Mirpurkhas Regions (Total Number of Schools: 209) Sr. # Name of District No. of Schools Sr. # Name of District No. of Schools 1 Karachi West 14 1 Dadu 14 2 Karachi South 20 2 Jamshoro 20 3 Karachi East 19 3 Hyderabad 20 4 Korangi 12 4 Tando Allah Yar 10 5 Karachi Central 14 5 Tando M. Khan 10 6 Malir 8 6 Matiari 17 Karachi Region Total Number of Schools 87 7 Badin 13 Sr. # Name of District No. of Schools 8 Sujawal 2 1 Mirpur Khas 7 Hyderabad Regioin 9 Thatta 6 2 Umerkot 2 Total Number of Schools 112 Regioin 3 Tharparkar 1 CLICK HERE TO ACCESS COMPLETE LIST OF Mirpur Mirpur Khas Total Number of Schools 10 SCHOOLS UNDER LOT # 1 LOT # 2 Larkana, Sukkur, and Benazirabad Regions (Total Number of Schools: 191) Sr. # Name of District No. of Schools Sr. # Name of District No. of Schools 1 Jacobabad 8 1 Sukkur 26 2 Kashmore 11 2 Ghotki 18 Sukkur 3 Shikarpur 8 Regioin 3 Khairpur 20 4 Larkana 32 Total Number of Schools 64 5 Shahdadkot 8 Sr. # Name of District No. of Schools Larkana Region Total Number of Schools 67 1 Naushahro Feroze 34 2 Sanghar 9 CLICK HERE TO ACCESS COMPLETE LIST OF Region 3 S. Benazir Abad 17 SCHOOLS UNDER LOT # 2 Shaheed Benazirabad Total Number of Schools 60 The contracting agency (RSU) reserves the right to change location of schools under any lot due to any reason whatsoever and such change may occur during pre or post award of contract (prior to scheduled delivery of goods in respective schools).