Update in Anaesthesia12
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Pharmacy Phacts in This Issue Pharmd Candidates Discuss Oral Health and Preventing Eye Strain at Work
Pharmacy Phacts In this issue PharmD candidates discuss Oral Health and Preventing Eye Strain At Work Oral Health Landon Forrest Stewart, PharmD Candidate 2021 Why is Oral Health important? Oral health is an important part of our overall health. Oral health issues can cause oral pain, increased costs in healthcare, and less productivity. Recent developments in oral hygiene have led to improved outcomes for patients. Many oral health issues are still prevalent today, but the good news is that many are preventable with daily healthy habits. Healthy habits are required to maintain good oral health and many oral health issues are still prevalent today. One of the most common oral health problems is decay in the tooth also known as a cavity. The outer layer of the tooth is a tough mineral layer called the enamel. Bacteria group together on teeth to form plaques that can erode the enamel and the deeper layers of your teeth, causing cavities. Cavities are present and untreated in up to 26% up American adults. (1) If left untreated, they can lead to pain in the tooth and more severe infections known as an abscess. Another common oral health problem is gum disease. When bacteria group together on the teeth, it causes the immune system to respond and causes inflammation in the mouth. The initial inflammation is called gingivitis and makes your gums swell and bleed more easily. Untreated gingivitis can progress to a more severe gum disease called periodontitis. Periodontitis can damage the structure that holds your teeth in place and is a common cause of tooth loss. -

Frequently Asked Questions (Faqs)
Frequently Asked Questions (FAQs) What is hyperbaric oxygen therapy? Commonly referred to as HBOT, hyperbaric oxygen therapy enhances the body’s natural healing process by delivering oxygen under pressure, which increases the oxygen content in the blood, plasma, cerebral spinal fluid, and other body tissues. There are two basic types of HBOT—hard HBOT and mild HBOT. With hard HBOT, treatments are delivered in a hard-sided chamber typically at pressures greater than 1.5 ATA and using 100% oxygen. 100% oxygen is extremely flammable; therefore, hard HBOT involves managing the risk of explosion. Another concern with hard HBOT is oxygen toxicity. While hard HBOT with 100% oxygen results in greater oxygen saturation in the tissues, many conditions respond better to mild HBOT. In clinical trials to date, there has been virtually no difference in clinical outcome between mild HBOT and hard HBOT. Mild HBOT refers to hyperbaric oxygen therapy at lower pressures, typically 1.5 ATA or below, and the use of an oxygen concentrator delivering 90-95% oxygen inside a portable soft-sided chamber. Mild HBOT has no known safety risks with fire or toxicity, and it is substantially less expensive. Our facility provides concentrated oxygen (90-95%) at 1.3 ATA—(Mild HBOT)a highly effective combination clinically, and without the risk of oxygen toxicity or explosion, as 100% oxygen is avoided. How should I expect mild HBOT to feel? You will be seated or lying down inside the chamber, relaxing comfortably in your own clothing, as you breathe concentrated oxygen (90-95% O2) through a facemask. -

Stratus 5 Oxygen Concentrator User Manual
USER MANUAL OXYGEN CONCENTRATOR CE 0123 v2.1 STR1005 User Manual Symbol Key MARK DEFINITION II Power on Power off Follow Instruction for Use No smoking Caution, consult accompanying documents. Class Ⅱ (Double Insulated) Type BF Applied Part CE certification mark 0123 AC Power Stacking Limit by Number This Way Up Fragile, handle with care Keep dry Temperature limit No open flames IIPP2211 IP21 Drip Proof Equipment Consult instructions for use Stand-by Warning, electricity 2 v2.1 STR1005 User Manual 3 v2.1 STR1005 User Manual SPECIAL NOTES • Please read this manual carefully before using this product and save it for future reference. • If you need assistance with this manual, Please contact your local DME or home health provider • The Stratus 5 is a prescription device. Use only the liter setting prescribed for you. • It is always recommended for critically ill patients to have a backup oxygen source in case of malfunction. • If patient experiences an adverse reaction contact physician or call 911 immediately. • In case of machine malfunction, contact the home medical equipment provider; do not attempt to disassemble the Stratus 5. • The Stratus 5 is not intended as life support, it is for supplemental oxygen use only. Patients with special needs may be unable to understand the alarm features and should be well supervised while using an oxygen concentrator. • The Stratus 5 is for single patient use. • Do not adjust the flowmeter float beyond the red line position. Long-term use out of range will reduce the efficiency of the oxygen generator. SAFETY NOTICE Please read the following information carefully before Operating the oxygen concentrator Warning Special attention should be paid to reducing the risk of fire when using oxygen therapy. -

Study Protocol
RESEARCH PROTOCOL Project Title A multicenter, single blind, randomized controlled trial of virucidal effect of Polyvinylpyrrolidone-Iodine on SARS-CoV-2 as well as safety of its application on nasopharynx & oropharynx of COVID-19 positive patients BMRC Reg. No: 38624012021 Page-1/17 Project Title A multicenter, single blind, randomized controlled trial of virucidal effect of Polyvinylpyrrolidone- Iodine on SARS-CoV-2 as well as safety of its application on nasopharynx & oropharynx of COVID- 19 positive patients. Summary Povidone Iodine (Iodine with water soluble polymer Polyvinylpyrolidone) or PVP-I is a proven and time trusted antiseptic agent having best possible (99.99%) virucidal effect in it‟s only 0.23% concentration, against all viruses including SARS-Co, MERS-CoV; even in SARS-COV-2 due to it‟s nonspecific mode of action for virus killing and having no resistance [1,2]. Corona virus is transmitted by/via respiratory droplets or aerosol, produced from sneezing or coughing of infected persons to healthy individual through mouth and nose mainly [5, 6]. The routes of entry of coronavirus in human body are mouth, nose and eye. PVP-I products for gargling the throat and spraying or washing the nose may have a preventive effect on COVID-19 and if it is proved in this study following human trial, this will be a landmark research in COVID-19 pandemic. In line of this, PVP-I containing oro-nasal spray, proposed Bangasafe, which should be regarded as PONS (Povidone Iodine oro-nasal spray) in this protocol, has been developed and proposed to use against corona virus disease. -
Viscotears Liquid Gel Later Than Four Weeks After First Opening
Viscotears® Liquid Gel carbomer (polyacrylic acid) Patient Information Leaflet This product will be called Viscotears in this leaflet. Please read this leaflet carefully before you start to use Viscotears. It contains important information. Keep the leaflet in a safe place because you may want to read it again. Do not share these eye drops with anyone else just in case you have an eye infection which you could pass on. If you have any other questions, or if there is something you don’t understand, please ask your pharmacist. If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist. In this leaflet: 1. What Viscotears is and what it’s used for 2. Things to consider before you start to use Viscotears 3. How to use Viscotears 4. Possible side effects 5. How to store Viscotears 6. Further information 1. What Viscotears is and what it’s used for Viscotears contains the active ingredient, carbomer (polyacrylic acid). Viscotears is used to make your eyes more comfortable when they feel dry. It is one of a group of eye drops called ‘artificial tears’. 2. Things to consider before you start to use Viscotears DO NOT use Viscotears if: If you are allergic to carbomer or any of the other ingredients of this medicine (listed in section 6). Take special care: In children and adolescents aged to 18 years, the safety and efficacy of Viscotears at the posology recommended in adults has been established by clinical experience, but no clinical trial data are available. -
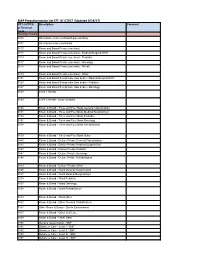
MAP Preauthorization List EFF: 8/1/2017 (Updated 8/24/17)
MAP Preauthorization List EFF: 8/1/2017 (Updated 8/24/17) CPT, HCPCS Description Comment or Revenue Code Revenue Codes 0100 All inclusive room and board plus ancillary 0101 All inclusive room and board 0110 Room and Board Private (one bed) 0111 Room and Board Private (one bed) - Medical/Surgical/GYN 0113 Room and Board Private (one bed) - Pediatric 0117 Room and Board Private (one bed) - Oncology 0118 Room and Board Private (one bed) - Rehab 0119 Room and Board Private (one bed) - Other 0121 Room and Board Semiprivate (two beds) - Medical/Surgical/GYN 0123 Room and Board Semiprivate (two beds) - Pediatric 0127 Room and Board Semiprivate (two beds) - Oncology 0128 Level 1 Rehab 0129 Level 2 Rehab - acute complex 0130 Room & Board - Three and Four Beds General Classification 0131 Room & Board - Three and Four Beds Medical/Surgical/Gyn 0133 Room & Board - Three and Four Beds Pediatric 0137 Room & Board - Three and Four Beds Oncology 0138 Room & Board - Three and Four Beds Rehabilitation 0139 Room & Board - Three and Four Beds Other 0140 Room & Board - Deluxe Private General Classification 0141 Room & Board - Deluxe Private Medical/Surgical/Gyn 0143 Room & Board - Deluxe Private Pediatric 0147 Room & Board - Deluxe Private Oncology 0148 Room & Board - Deluxe Private Rehabilitation 0149 Room & Board - Deluxe Private Other 0150 Room & Board - Ward General Classification 0151 Room & Board - Ward Medical/Surgical/Gyn 0153 Room & Board - Ward Pediatric 0157 Room & Board - Ward Oncology 0158 Room & Board - Ward Rehabilitation 0159 Room & Board - -

Formulary Drug List
AMLODIPINE ORAL SUSPENSION Products Affected Step 2: • KATERZIA 1 MG/ML ORAL SUSPENSION Details Criteria PRIOR CLAIM FOR GENERIC AMLODIPINE TABLETS WITHIN THE PAST 120 DAYS. 1 ANTIBACTERIALS (EENT) Products Affected Step 2: • BESIVANCE 0.6 % EYE DROPS,SUSPENSION Details Criteria PRIOR CLAIM FOR FORMULARY VERSION OF CIPROFLOXACIN OPHTHALMIC OR OFLOXACIN OPHTHALMIC DROPS WITHIN THE LAST 120 DAYS. 2 ANTIDEPRESSANTS Products Affected Step 2: • FETZIMA 120 MG CAPSULE,EXTENDED RELEASE CAPSULE,EXTENDED RELEASE • FETZIMA 40 MG • FETZIMA 20 MG (2)-40 MG (26) CAPSULE,EXTENDED RELEASE CAPSULE,EXTENDED RELEASE,24 • FETZIMA 80 MG HR,DOSE PACK CAPSULE,EXTENDED RELEASE • FETZIMA 20 MG Details Criteria PRIOR CLAIM FOR TRINTELLIX AND VIIBRYD WITHIN THE PAST 365 DAYS. 3 ANTIPSYCHOTIC AGENTS Products Affected Step 2: • aripiprazole 10 mg disintegrating tablet • FANAPT 4 MG TABLET • aripiprazole 15 mg disintegrating tablet • FANAPT 6 MG TABLET • asenapine 10 mg sublingual tablet • FANAPT 8 MG TABLET • asenapine 2.5 mg sublingual tablet • SECUADO 3.8 MG/24 HOUR • asenapine 5 mg sublingual tablet TRANSDERMAL 24 HOUR PATCH • CAPLYTA 42 MG CAPSULE • SECUADO 5.7 MG/24 HOUR • clozapine 100 mg disintegrating tablet TRANSDERMAL 24 HOUR PATCH • clozapine 12.5 mg disintegrating tablet • SECUADO 7.6 MG/24 HOUR • clozapine 150 mg disintegrating tablet TRANSDERMAL 24 HOUR PATCH • clozapine 200 mg disintegrating tablet • VERSACLOZ 50 MG/ML ORAL • clozapine 25 mg disintegrating tablet SUSPENSION • FANAPT 1 MG TABLET • VRAYLAR 1.5 MG (1)-3 MG (6) • FANAPT 10 MG TABLET CAPSULES -

Handbook ESRA
TECHNIQUES HEAD & NECK 4 Intracranial surgery p. 3 Eye blocks p. 5 Face anatomy p. 16 Face particularity p. 23 Ophtalmic nerve blocks p. 27 Maxillary nerve blocks p. 33 Mandibular nerve blocks p. 46 THORAX & ABDOMEN 50 Epidural anaesthesia in Cardio-thoracic surgery p. 50 Ilioinguinal-Iliohypogastric block p. 55 Peri-umbilical & Rectus sheath block p. 57 Pudendal block p. 58 UPPER LIMB 61 Choice of a technique p. 61 Brachial plexus anatomy p. 65 Interscalen block p. 68 Supraclavicular blocks p. 73 Infraclavicular blocks p. 80 Axillary block p. 83 LOWER LIMB 90 Lumbar plexus block p. 90 Iliofascial block p. 100 Obturator block p. 102 Sciatic blocks o Sciatic blocks - parasacral nerve approach p. 109 o Sciatic blocks - posterior popliteal approach p. 115 Ankle blocks p. 119 AXIAL BLOCKS 123 Lumbar epidural p. 123 OBSTETRICS AXIAL BLOCKS 126 Epidural p. 126 PERIPHERAL BLOCKS Pudendal block p. 58 2 Aknowledgement The provenience of the materials included in this handbook is from the Learning Zone on the official site of “European Society of Regional Anesthesia and Pain Therapy”. http://www.esra-learning.com/ 2007 3 HEAD & TABLE OF CONTENTS NECK • Intracranial surgery • Eye blocks • Face anatomy • Face particularity • Ophtalmic nerve blocks • Maxillary nerve blocks • Mandibular nerve blocks • Cervical plexus blocks HEAD & INTRACRANIAL SURGERY NECK Paul J. Zetlaoui, M.D. Kremlin-Bicetre - France In intra-cranial neurosurgery, scalp infiltration aims to prevent systematic and cerebral hemodynamic variations, contemporary of skin incision. The potential morbidity of these hypertension-tachycardia episodes, even in patients profoundly anaesthetized, is secondary in the increase of the cerebral blood flow and in its deleterious consequences on intra-cranial pressure in these compromised patients. -

E-Cigarette Use in Patients Receiving Home Oxygen Therapy
FOCUSED REVIEW E-cigarette use in patients receiving home oxygen therapy Yves Lacasse MD MSc FRCP1,2, Martin Légaré MD FRCP3, François Maltais MD FRCP1,2 Y Lacasse, M Légaré, F Maltais. E-cigarette use in patients receiving La cigarette électronique chez les patients sous home oxygen therapy. Can Respir J 2015;22(2):83-85. oxygénothérapie à domicile Current smokers who are prescribed home oxygen may not benefit from the therapy. In addition to being an obvious fire hazard, there is some evi- Il se peut que les fumeurs qui se font prescrire une oxygénothérapie à domi- dence that the physiological mechanisms by which home oxygen is cile ne profitent pas de ce traitement. Sans compter que le tabagisme pose believed to operate are inhibited by smoking. Although their effectiveness un risque d’incendie évident, certaines données probantes indiquent qu’il is yet to be demonstrated, electronic cigarettes (e-cigarettes) are often inhibe les mécanismes physiologiques par lesquels l’oxygénothérapie à regarded as an aid to smoking cessation. However, several burn accidents domicile fonctionnerait. Même si son efficacité reste à démontrer, la ciga- in e-cigarette smokers receiving home oxygen therapy have also been rette électronique (vapoteuse) est souvent perçue comme une aide au reported, leading Health Canada to release a warning of fire risk to oxygen sevrage du tabagisme. Cependant, plusieurs incidents de brûlure chez des therapy patients from e-cigarettes. It is the authors’ position that patients vapoteurs sous oxygénothérapie à domicile ont été déclarés, ce qui a incité receiving oxygen should definitely not use e-cigarettes. -

A Potential Alternative Orodispersible Formulation to Prednisolone Sodium Phosphate Orally Disintegrating Tablets
pharmaceutics Article A Potential Alternative Orodispersible Formulation to Prednisolone Sodium Phosphate Orally Disintegrating Tablets Essam A. Tawfik 1,2,* , Mariagiovanna Scarpa 2, Hend E. Abdelhakim 2 , Haitham A. Bukhary 2,3, Duncan Q. M. Craig 2 , Susan A. Barker 4 and Mine Orlu 2 1 National Center for Pharmaceutical Technology, Life Science and Environment Research Institute, King Abdulaziz City for Science and Technology, P.O. Box 6086, Riyadh 11442, Saudi Arabia 2 Department of Pharmaceutics, UCL School of Pharmacy, University College London, 29-39 Brunswick Square, London WC1N 1AX, UK; [email protected] (M.S.); [email protected] (H.E.A.); [email protected] (H.A.B.); [email protected] (D.Q.M.C.); [email protected] (M.O.) 3 Department of Pharmaceutics, College of Pharmacy, Umm Al-Qura University, Makkah 24381, Saudi Arabia 4 Medway School of Pharmacy, The Universities of Greenwich and Kent at Medway, Anson Building Central Avenue, Chatham, Kent ME4 4TB, UK; [email protected] * Correspondence: etawfi[email protected] Abstract: The orally disintegrating tablet (ODT) has shown vast potential as an alternative oral dosage form to conventional tablets wherein they can disintegrate rapidly (≤30 s) upon contact with saliva fluid and should have an acceptable mouthfeel as long as their weight doesn’t exceed 500 mg. However, owing to the bitterness of several active ingredients, there is a need to find a suitable alternative to ODTs that maintains their features and can be taste-masked more simply and inexpensively. Therefore, electrospun nanofibers and solvent-cast oral dispersible films (ODFs) are used in this study as potential OD formulations for prednisolone sodium phosphate (PSP) that is Citation: Tawfik, E.A.; Scarpa, M.; commercially available as ODTs. -

Can Pilocarpine Eye Drops Be Used to Treat Dry Mouth?
Page 1 of 5 Question: Can pilocarpine eye drops be used to treat dry mouth? July 2019 Summary Pilocarpine is not recommended as a first line treatment for dry mouth (xerostomia). However, saliva stimulants are generally more effective than saliva substitutes (unless a main salivary duct is blocked) 1, and are preferred by patients.2 Other saliva stimulants such as sugar-free chewing gum should be tried prior to the initiation of pilocarpine. Pilocarpine tablets (Salagen®) are unlicensed in Ireland but are licensed in the UK for the treatment of dry mouth following irradiation for head and neck cancer and for patients with Sjögren's syndrome.3 There is limited information available to support the oral use of pilocarpine eye drops as an alternative. The use of pilocarpine eye drops to treat a dry mouth is unlicensed. For cost/reimbursement reasons, pilocarpine eye drops administered orally are favored over the use of Salagen® oral tablets (unlicensed). A recommended dose of 5mg - 10mg three times daily of oral pilocarpine tablets is approximately equivalent to pilocarpine 4% w/v, 3 - 5 drops (6mg-10mg) taken orally three times daily. 1 - 6 Palliative Meds Info: Terms and Conditions The information outlined above is intended for healthcare professionals only. The information outlined above is believed to accurately reflect the medical literature at the time of writing. Healthcare professionals must use their own judgment to determine the accuracy and relevance of the information. See www.olh.ie for full terms and conditions. Page 2 of 5 Availability Pilocarpine tablets are indicated for the treatment of dry mouth following irradiation for head and neck cancer and for dry mouth and dry eyes in Sjogren’s syndrome. -

Florida Special Needs Registry Registration Information - Osceola County
Florida Special Needs Registry Registration Information - Osceola County Instructions: Complete this form and fax or mail it to Osceola County to register an individual for the Florida Special Needs Registry. This form is not required if you have already registered on line. Required fields are indicated with an asterisk (*). Mail: Osceola County Special Needs Registry Fax: (407) 742-9022 2586 Partin Settlement Road Kissimmee, FL 34744 PERSONAL INFORMATION ABOUT THE REGISTRANT *First Name Middle Name *Last Name Suffix *Birth Date *Gender (select only one) Male Female Transgender Non-Binary Prefer Not To Provide *Height Feet: Inches: *Weight (pounds) Living Situation (select only one) Live alone Live with relative or Other living situation caregiver *Primary Language Secondary Language Veteran Yes No Last 4 digits of SSN Email Address Are you completing this form on behalf of the Family Member Caregiver Neighbor Friend registrant? If so, please indicate your relationship to the registrant (select only Home Health Care County Emergency County Health DOH State Staff one) Provider Management Staff Department Staff ADDRESS FOR THE REGISTRANT (physical address is required) *Physical Address (cannot be a PO Box) *Physical City *Physical State FL *Physical Zip Code Name of Complex, Subdivision or Mobile Home Park Is the home at this address a mobile home? Yes No Is the home at this address a highrise or Yes No multi-story home? Does this home have stairs? Yes No Is there a gate that requires a code to enter? Yes No Do you live at this address