CTRI Trial Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

8. Commission Will Not Accept New Hard Copies of Entire
Staff Selection Commission (Karnataka – Kerala Region) First Floor, ‘E’ – Wing, KendriyaSadan, Koramangala,Bangalore – 560 034. Helplines: 080-25502520/0-9483862020 Website: http:\\ssckkr.kar.nic.in No.S.600001/15/2014-Sel. Dated, the.,25th September, 2017. Subject: Recruitment to the post ofJunior Engineer (Quality Assurance).in Controllarate of Quality Assurance (Electronics), In Dept. of Defence Production, M/o Defence- Cat. Code No.KK10217 of Advt. No. KKR-1/2017 - regarding. ……………………….. Staff Selection Commission (KKR), completedscrutiny for the above captioned post, after receipt of hard copies of application with supporting documents from candidates, in response to Advertisement No. KKR-01/2017. 2. Details of ineligible applicants applied online,including the details of those candidates from whom hard copy has not been received on or before 07.07.2017 in this Regional Office,is given below with reason mentioned against their names. Essential Qualifications for the post (i) Bachelor’s Degree in science with one of the subjects at Graduate Level viz., physics, Computer Science, Electronics from a recognised University OR Diploma in Engineering or Technology in one of the disciplines viz., Electronics, Electrical, Computers, Information Technology, Electronics & Telecommunication from a recognised University or State Board or Technical Education. (ii) One Year experience in Production or Development or Quality Assurance in the fields of Electrical or Electronics or Computer from a recognised Organisation or Undertaking. Note: As per clarification received from Dte. General of Quality Assurance, D/o Defence Production, M/o Defence vide letter No. A/92163/Rectt. Gr.(B)/SSC/DGQA/Veh/Adm-7B dated 23..02.2016, candidates who have obtained minimum one year’s experience certificate in the relevant field from an Organisation by the Government of India/concerned State Government or an Undertaking can be considered eligible. -
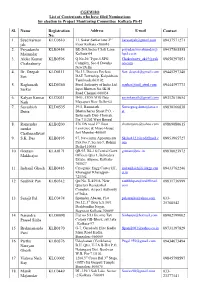
CGEWHO List of Contestants Who Have Filed Nominations for Election in Project Monitoring Committee Kolkata Ph-II
CGEWHO List of Contestants who have filed Nominations for election in Project Monitoring Committee Kolkata Ph-II Sl. Name Registration Address E-mail Contact No. 1. Syed kaiwan KLC0610 11, Sakur Sarkar lane 2nd [email protected] 09437571271 jah Floor Kolkata -700016 2. Priyadarshi KLB0454 BE-260,Sector I Salt Lane priyadarshimahumdar@ 09437565555 Majumdar Kolkata-64 hpcl.co.in 3. Aloke Kumar KLB0596 Q.No.20/ Type-5,SPG Chakraborty_ak19@yah 09650297851 Chakraboriy Complex, Sec-8 Dwarka oo.com New Delhi 4. Dr. Deepak KLD0111 No.13, Doctors Enclave [email protected] 09445297340 Sen DAE Township, Kalpakkam Tamilnadu 603102 5. Raghunath KLD0360 Steel Authority of India Ltd. rsarkar@sail_steel.com 09444397773 Sarkar Ispat Bhawan No.5K H Road Chennai 600034 6. Kalyan Kumar KLC0031 D-9E, DDA MIG flats [email protected] 09312615654 Nath Mayapuri New Delhi-64 7. Suvashish KLD0535 39/5, Ramanath [email protected] 09830166838 Dutta Bhattacharya Street P.O. - et Belurmath Dist- Howrah. Pin 711202 West Bengal 8. Ramendra KLB0200 276 DN road 2nd floor [email protected] 09869080615 sundar Lawrence & Mayo House Chattopadhyay fort Mumbai-400001 9. S.K. Das KLB0195 97, Naveentm Appartments [email protected] 09953965727 Plot No.7, Sector-9, Rohini om Delhi-110085 10. Goutam KLA0171 QR.95, BL-16,Central Govt. [email protected] 09830023972 Mukherjee Officers Qtrs 1, Belveders Extate, Alipore, Kolkata- 700027 11. Indranil Ghosh KLB0485 Cryogenic Engg Center IIT, [email protected] 09433762246 Kharagpur Kharagpur- et.in 721302 12. Saubhik Pan KLB0342 Qtr.No. R-459/A, New saubhikpan@rediffmail. 09831736999 Quarters Residential com Complex, Airport Authority of India, 13. -
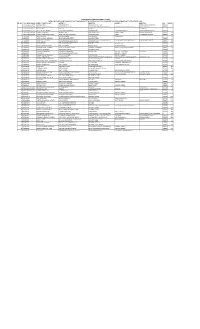
IEPF-SHARES-2020.Pdf
INTERNATIONAL COMBUSTION (INDIA) LIMITED DETAILS OF EQUITY SHARES PROPOSED TO BE TRANSFERRED TO THE INVESTOR EDUCATION AND PROTECTION FUND AUTHORITY AFTER 5TH OCTOBER, 2020 SR. NO. FOLIO NO/DPID&CLID NAME OF SHAREHOLDER ADDRESS1 ADDRESS2 ADDRESS3 ADDRESS4 PIN SHARES 1 1201060800108747 SHIVAM KAPRI SHIVAM KUTEER JEEVAN JYOTI COLONY SATNA MADHYA PRADESH 485001 5 2 1201910100265850 RAJESH KESHAVLAL PATEL H.NO. 544, PATELVAS, GHANSHYAM NAGAR, MANEKPUR TA-MANSA, DIST-GANDHI NAGAR MANSA GUJARAT 382845 5 3 1203160000196267 KIRAN GARG HNO. 28 KAJIPURA BHOPAL MP . 50 4 1203320001312751 PREETI ANANT SAWANT 1/24 SAHAJIVAN NIWAS KOKAN NAGAR J M ROAD BHANDUP MUMBAI MAHARASHTRA 400078 5 5 1301760000506195 SAMPAT LAL BAFNA NOKHA ROAD OPP. BAFNA TRUST GANGASAHAR BIKANER RAJASTHAN 334401 10 6 1304140001710072 JIGNA CHANDRAKANT RAVAL B 101 VIJAY COMPLEX NEAR VASNA BUS STOP VASNA AHMEDABAD GUJARAT 380007 20 7 A0000353 SAROJ ADVANI 182 NEELAMBER PEDDER ROAD MUMBAIBAY 0 26 8 A0000374 SURESH KUMAR AGARWAL 58,SHYAM BAZAR STREET KOLKATA 700004 700004 1 9 A0000385 FARID FAZAL ALLANA C/O KOHONOOR COLLECTIONS UNIQUE HOUSE,25 S.A.BRELVI ROAD OPP.BOMBAY SAMACHAR PRESS FORT.MUMBAI 400001 400001 50 10 A0000401 SAROJ ADVANI 182 NEELAMBER PEDDER ROAD MUMBAI 400401 400401 95 11 A0000451 A R AGRAWAL 25/42,KANKALI PARA RAIPUR (M.P) 492001 492001 1 12 A0000469 SAROJ CHANDUR ADVANI 182 NEELAMBER PEDDAR ROAD MUMBAI 400026 400026 29 13 A0000538 RAM PARAKASH AGARWAL 5 9 1121 F 43 FIRST FLOOR AGARWAL CHAMBERS KING KOTI HYDERABAD 500001 500001 10 14 B0000348 BUDH RAJ BHANSALI -

Annexure 1B 18416
Annexure 1 B List of taxpayers allotted to State having turnover of more than or equal to 1.5 Crore Sl.No Taxpayers Name GSTIN 1 BROTHERS OF ST.GABRIEL EDUCATION SOCIETY 36AAAAB0175C1ZE 2 BALAJI BEEDI PRODUCERS PRODUCTIVE INDUSTRIAL COOPERATIVE SOCIETY LIMITED 36AAAAB7475M1ZC 3 CENTRAL POWER RESEARCH INSTITUTE 36AAAAC0268P1ZK 4 CO OPERATIVE ELECTRIC SUPPLY SOCIETY LTD 36AAAAC0346G1Z8 5 CENTRE FOR MATERIALS FOR ELECTRONIC TECHNOLOGY 36AAAAC0801E1ZK 6 CYBER SPAZIO OWNERS WELFARE ASSOCIATION 36AAAAC5706G1Z2 7 DHANALAXMI DHANYA VITHANA RAITHU PARASPARA SAHAKARA PARIMITHA SANGHAM 36AAAAD2220N1ZZ 8 DSRB ASSOCIATES 36AAAAD7272Q1Z7 9 D S R EDUCATIONAL SOCIETY 36AAAAD7497D1ZN 10 DIRECTOR SAINIK WELFARE 36AAAAD9115E1Z2 11 GIRIJAN PRIMARY COOPE MARKETING SOCIETY LIMITED ADILABAD 36AAAAG4299E1ZO 12 GIRIJAN PRIMARY CO OP MARKETING SOCIETY LTD UTNOOR 36AAAAG4426D1Z5 13 GIRIJANA PRIMARY CO-OPERATIVE MARKETING SOCIETY LIMITED VENKATAPURAM 36AAAAG5461E1ZY 14 GANGA HITECH CITY 2 SOCIETY 36AAAAG6290R1Z2 15 GSK - VISHWA (JV) 36AAAAG8669E1ZI 16 HASSAN CO OPERATIVE MILK PRODUCERS SOCIETIES UNION LTD 36AAAAH0229B1ZF 17 HCC SEW MEIL JOINT VENTURE 36AAAAH3286Q1Z5 18 INDIAN FARMERS FERTILISER COOPERATIVE LIMITED 36AAAAI0050M1ZW 19 INDU FORTUNE FIELDS GARDENIA APARTMENT OWNERS ASSOCIATION 36AAAAI4338L1ZJ 20 INDUR INTIDEEPAM MUTUAL AIDED CO-OP THRIFT/CREDIT SOC FEDERATION LIMITED 36AAAAI5080P1ZA 21 INSURANCE INFORMATION BUREAU OF INDIA 36AAAAI6771M1Z8 22 INSTITUTE OF DEFENCE SCIENTISTS AND TECHNOLOGISTS 36AAAAI7233A1Z6 23 KARNATAKA CO-OPERATIVE MILK PRODUCER\S FEDERATION -

INDIAN OVERSEAS BANK Page 1 of 41 Clerical to Officer As on 31.12
INDIAN OVERSEAS BANK Human Resources Development Department Page 1 of 41 No 763, Anna Salai , Chennai-600002 Clerical to Officer as on 31.12.2014 - Post :JM I - PROMOTEE Cycle No SL No UR or Reserved for Emp No Emp Name Recruitment Dt Caste_Category Reserved 1 1 UR 5665 DEEP NARAYAN SINGH 15.09.1973 UR UR 1 2 UR 5653 DEVARAJU T 01.03.1974 SC SC-1 1 3 UR 5758 RAMKISHAN 14.05.1974 SC SC-2 1 4 UR 5812 PALAI D C 07.10.1974 UR UR 1 5 UR 5772 BALAUR SINGH 15.11.1974 SC SC-3 1 6 UR 8915 LATABEN HARSHADKUMAR BRAH 17.01.1975 SC UR 1 7 SC-1 8972 SHAILENDRAKUMAR SAXENA 08.02.1975 UR UR 1 8 UR 9404 PRADEEP KUMAR SANYAL 21.05.1975 UR UR 1 9 UR 5970 BEER SAIN 09.06.1975 UR UR 1 10 UR 9402 SRIRAM V 16.06.1975 UR UR 1 11 UR 5948 GNANA DOSS D 01.09.1975 UR UR 1 12 UR 9681 GUNASEKARAN G 15.09.1975 SC UR 1 13 UR 6070 KISHORI LAL 21.10.1975 SC SC-4 1 14 ST-1 9903 JITENDRA RAJDEV S 17.11.1975 UR UR 1 15 SC-2 5993 BOPALIA D T 10.12.1975 UR UR 1 16 UR 6034 BARICK S K 29.12.1975 UR UR 1 17 UR 6057 SHYAM SUNDER M 10.03.1976 UR UR 1 18 UR 10324 MAHENDAR SINGH VARMA 26.03.1976 UR UR 1 19 UR 6136 APPA RAO G 15.04.1976 SC SC-5 1 20 SC-3 6068 KANNAN CT L 20.04.1976 UR UR 1 21 UR 10462 MAN MOHAN SHARMA 03.05.1976 UR UR 1 22 UR 6231 SATNAM SINGH 06.05.1976 SC SC-6 1 23 UR 6105 RAJ KISHORE 11.05.1976 SC SC-7 1 24 UR 6164 BHAGWAN DASS 03.06.1976 SC SC-8 1 25 UR 6436 RAMESH LAL 07.06.1976 SC SC-9 1 26 UR 10726 SREEDHARAMOORTHY E 05.07.1976 UR UR 1 27 SC-4 6201 SRIDHARA KONDANGIRI 12.07.1976 SC SC-10 1 28 ST-2 6282 DAYA RAM 28.07.1976 SC SC-11 1 29 UR 6374 KALYAN CHAND -
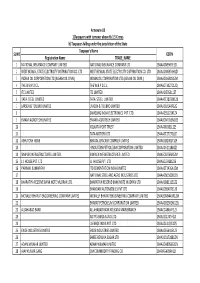
FINAL DISTRIBUTION.Xlsx
Annexure-1B 1)Taxpayers with turnover above Rs 1.5 Crores b) Taxpayers falling under the jurisdiction of the State Taxpayer's Name SL NO GSTIN Registration Name TRADE_NAME 1 NATIONAL INSURANCE COMPANY LIMITED NATIONAL INSURANCE COMPANY LTD 19AAACN9967E1Z0 2 WEST BENGAL STATE ELECTRICITY DISTRIBUTION CO. LTD WEST BENGAL STATE ELECTRICITY DISTRIBUTION CO. LTD 19AAACW6953H1ZX 3 INDIAN OIL CORPORATION LTD.(ASSAM OIL DIVN.) INDIAN OIL CORPORATION LTD.(ASSAM OIL DIVN.) 19AAACI1681G1ZM 4 THE W.B.P.D.C.L. THE W.B.P.D.C.L. 19AABCT3027C1ZQ 5 ITC LIMITED ITC LIMITED 19AAACI5950L1Z7 6 TATA STEEL LIMITED TATA STEEL LIMITED 19AAACT2803M1Z8 7 LARSEN & TOUBRO LIMITED LARSEN & TOUBRO LIMITED 19AAACL0140P1ZG 8 SAMSUNG INDIA ELECTRONICS PVT. LTD. 19AAACS5123K1ZA 9 EMAMI AGROTECH LIMITED EMAMI AGROTECH LIMITED 19AABCN7953M1ZS 10 KOLKATA PORT TRUST 19AAAJK0361L1Z3 11 TATA MOTORS LTD 19AAACT2727Q1ZT 12 ASHUTOSH BOSE BENGAL CRACKER COMPLEX LIMITED 19AAGCB2001F1Z9 13 HINDUSTAN PETROLEUM CORPORATION LIMITED. 19AAACH1118B1Z9 14 SIMPLEX INFRASTRUCTURES LIMITED. SIMPLEX INFRASTRUCTURES LIMITED. 19AAECS0765R1ZM 15 J.J. HOUSE PVT. LTD J.J. HOUSE PVT. LTD 19AABCJ5928J2Z6 16 PARIMAL KUMAR RAY ITD CEMENTATION INDIA LIMITED 19AAACT1426A1ZW 17 NATIONAL STEEL AND AGRO INDUSTRIES LTD 19AAACN1500B1Z9 18 BHARATIYA RESERVE BANK NOTE MUDRAN LTD. BHARATIYA RESERVE BANK NOTE MUDRAN LTD. 19AAACB8111E1Z2 19 BHANDARI AUTOMOBILES PVT LTD 19AABCB5407E1Z0 20 MCNALLY BHARAT ENGGINEERING COMPANY LIMITED MCNALLY BHARAT ENGGINEERING COMPANY LIMITED 19AABCM9443R1ZM 21 BHARAT PETROLEUM CORPORATION LIMITED 19AAACB2902M1ZQ 22 ALLAHABAD BANK ALLAHABAD BANK KOLKATA MAIN BRANCH 19AACCA8464F1ZJ 23 ADITYA BIRLA NUVO LTD. 19AAACI1747H1ZL 24 LAFARGE INDIA PVT. LTD. 19AAACL4159L1Z5 25 EXIDE INDUSTRIES LIMITED EXIDE INDUSTRIES LIMITED 19AAACE6641E1ZS 26 SHREE RENUKA SUGAR LTD. 19AADCS1728B1ZN 27 ADANI WILMAR LIMITED ADANI WILMAR LIMITED 19AABCA8056G1ZM 28 AJAY KUMAR GARG OM COMMODITY TRADING CO. -

Tamil Nadu Government Gazette
© [Regd. No. TN/CCN/467/2012-14. GOVERNMENT OF TAMIL NADU [R. Dis. No. 197/2009. 2020 [Price : Rs. 16.80 Paise. TAMIL NADU GOVERNMENT GAZETTE PUBLISHED BY AUTHORITY No.43] CHENNAI, WEDNESDAY, OCTOBER 21, 2020 Aippasi 5, Saarvari, Thiruvalluvar Aandu – 2051 Part VI—Section 4 Advertisements by private individuals and private institutions CONTENTS PRIVATE ADVERTISEMENTS Pages. Change of Names .. 949-989 Notices .. 989-990 NOTICE NO LEGAL RESPONSIBILITY IS ACCEPTED FOR THE PUBLICATION OF ADVERTISEMENTS REGARDING CHANGE OF NAME IN THE TAMIL NADU GOVERNMENT GAZETTE. PERSONS NOTIFYING THE CHANGES WILL REMAIN SOLELY RESPONSIBLE FOR THE LEGAL CONSEQUENCES AND ALSO FOR ANY OTHER MISREPRESENTATION, ETC. (By Order) Commissioner of Stationery and Printing. CHANGE OF NAMES 13282. I, Seeli David, wife of Thiru A. David Rajakumar, 13285. I, L. Prakash, son of Thiru A. Lakshmanan, born on born on 29th April 1964 (native district: Thoothukkudi), 9th June 1999 (native district: Madurai), residing at No. 263, residing at No. 2/44A, N.G.O. Colony, Satchiyapuram, Ayyanar Nagar, Kallandhiri Post, Thoppulampatti, Madurai- Sengamalanatchiyarpuram, Sivakasi Taluk, Virudhunagar-626 124, shall henceforth be 625 301, shall henceforth be known as L. NALLUCHAMY known as A. PACKIASEELI SUGANTHY L. PRAKASH SEELI DAVID Madurai, 12th October 2020. Virudhunagar, 12th October 2020. 13286. I, K Muralikrishnan, son of Thiru M Kalyanarengan, 13283. My son, T. Alen Durai, son of Thiru A. Thinakaran, born on 5th January 1999 (native district: Pondicherry), born on 4th Septemer 2004 (native district: Tirunelveli), residing at Old No. 4-5, New No. 361-1, Annaimeenakshi residing at Old No. 26, New No. 27, South Krishnan Street, Kalaivanar Nagar, Vadipatti Taluk, Madurai-625 501, Kovil 4th Lane, Madurai-625 001, shall henceforth be shall henceforth be known as T. -
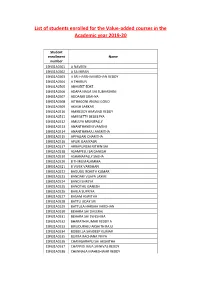
List of Students Enrolled for the Value-Added Courses in the Academic Year 2019-20
List of students enrolled for the Value-added courses in the Academic year 2019-20 Student enrollment Name number 19N31A0501 A NAVEEN 19N31A0502 A SAI KIRAN 19N31A0503 A SRI HARSHAVARDHAN REDDY 19N31A0504 A THARUN 19N31A0505 ABHIJEET EDKE 19N31A0506 ADAPA NAGA SAI SUBHASHINI 19N31A0507 ADDANKI GRAHYA 19N31A0508 AITHAGONI ANJALI GOUD 19N31A0509 AKASH SARKAR 19N31A0510 AMIREDDY ARAVIND REDDY 19N31A0511 AMRISETTY DEDEEPYA 19N31A0512 AMULYA MUNIPALLY 19N31A0513 ANANTHANENI VAMSHI 19N31A0514 ANANTHARAJU AASRITHA 19N31A0515 APPAGARI CHARITHA 19N31A0516 APURI GAMYASRI 19N31A0517 ARRAPUNEM NITHIN SAI 19N31A0518 ASAMPELLI SAIGANESH 19N31A0519 ASHANAPALLY SNEHA 19N31A0520 B THIRUMALAMMA 19N31A0521 B VIVEK VARDHAN 19N31A0522 BADUGU ROHITH KUMAR 19N31A0523 BANDARI VIJAYA LAXMI 19N31A0524 BANDI SHREYA 19N31A0525 BANOTHU GANESH 19N31A0526 BARLA SUPRIYA 19N31A0527 BASAM ASWITHA 19N31A0528 BATTU UDAY SRI 19N31A0529 BATTULA HARSHA VARDHAN 19N31A0530 BEHARA SAI DHEERAJ 19N31A0531 BEHARA SAI SWESHIKA 19N31A0532 BHARATH KUMAR REDDY A 19N31A0533 BIRUDURAJU AKSHITH RAJU 19N31A0534 BOBBILLA SANDEEP KUMAR 19N31A0535 BURRA RACHANA PRIYA 19N31A0536 CHANIGARAPU SAI AKSHITHA 19N31A0537 CHAPPIDI RAJA SRINIVAS REDDY 19N31A0538 CHENNALA MAHESHWAR REDDY 19N31A0539 CHEVVAKULA VIZNETHA RANI 19N31A0540 CHIKYALA KAVYA 19N31A0541 CHILUKA GANESH 19N31A0542 CHILUVURI SAI TEJA 19N31A0543 CHIMMULA HARSHITH REDDY 19N31A0544 CHINDALUR SRIDEVI 19N31A0545 CHINTALA RETHIKA REDDY 19N31A0546 LUCKY REDDY MEGHANA REDDY 19N31A0547 CHINTHALAPATI RISHIKA 19N31A0548 CHINTHIREDDY PRADEEP REDDY -

All Technical Sessions (16-12-2017)
ALL TECHNICAL SESSIONS (16-12-2017) Panel 1- State of the Discipline of Political Science in India Chair: Prof. Amiya Kumar Pricha, Behrampur University, Behrampur (Odisha) Co-chair: Prof. Poonam Bawa, J.N. Vyas University, Jodhpur (Rajasthan) Key Speaker: Prof. Kalpana Agrahari, Kumaun University, Nainital (Uttarakhand) Discussant: Dr. R. Venkatesh, University of Madras, Chennai (Tamilnadu) 1. Political Decentralization: A Theoretical Perception Dr. Adulapuram Thirupathi 2. A Common Man’s Perspective of Political Science: Dr. Bikash Chandra Dash Indian Experience 3. Challenges for the future of the Discipline of Public Dr. Swanamayee Tripathi Administration 4. Nature and Significance of John Rawls theory of DR.ASHOK S. ANIKIVI Justice 5. Nature and Significance of John Rawls theory of Dr. Bindushree Mishra Justice 6. Approa ches to the Study of Indian Political Thought Prof. S.A. Palekar 7. The Illiberal Liberalism: Post-Colonial Capitalism Atriya Dey and the Politics of Marginalization 8. Debating Political Anthropology in a Post-colonial Parvez Alam Society 9. Restructuring of the Indian State with the incursion Hushiar Singh of Neoliberal Politics 10. Indian Welfare state; Issues and Challenges Dr. Hariprasad Arukonda 11. Hannah Arendt’s “Human Condition” and Politics Prof. Sonali Singh in Emerging India 12. Public Administration in the Era of Globalization in Dr. Bhageerathi N. Naik India 13. Feminism, politics and Sexuality: Revisiting Dr. Amit Sharma Women’s journey from being self-effacing to self- assertive 1 Panel: 2 - Political Thought in India - Traditional and Modern (Hindi) Chair: Prof. Jitendra Narayan, L N Mithila University, Darbhanga (Bihar) Co-chair: Prof. Pawan Kumar Sharma, Atal Bihari Vajpayee Hindi University, Bhopal (M.P.) Key Speaker: Dr. -

Contact Details of Officers of Overseas Employment Division
Contact details of officers of Overseas Employment Division S.No. Name and Designation Contact Details 1 Shri Sanjay Bhattacharyya, Secretary (CPV & OIA) & Tel-49018134 Appellate Authority Fax:49018135 Email:[email protected] Room No.2013-C Wing 2 Shri Inderjeet Singh, PPS Tel-49018134 Fax:49018135 Email:[email protected] Room No.2011 C wing 3 Shri C.H Ramachandran, PPS Tel-49018134 Fax:49018135 Email:[email protected] Room No.2011 C wing 4 Shri K Srinivasarao, PS Tel-49018134 Fax:49018135 Email:[email protected] Room No.2068 B Wing 5 Shri Kapil Bansal, Steno Tel-49018134 Fax:49018135 Email:[email protected] Room No.2011 C wing 6 Shri Yogeshwar Sangwan, Joint Secretary/ PGE Tel-26874250 Email: [email protected] Room No.1013-14 7 Ms Binda Rawat, Sr.PPS Tel-26874250 Email: [email protected] Room No.1009 8 Shri Karan Singh Bangari, PPS Tel-26874250 Email: [email protected] Room No.1009 9 Shri Sumit Srivastava, Jr. Secretariat Assistant Tel-24197945 Email:[email protected] OE-II Section 10 Shri Raj Kumar Singh, Director Tel-24197994 Email: [email protected] Room No.933 11 Shri Ram Avtar Meena, US Tel-24197993 Email: [email protected] Room No.1021 12 Shri Saurabh Suman, ASO Tel-24197931 Room No.1035 OE-III Section 13 Shri Pramil Gupta, Director Tel-24197914 Email: [email protected] Room No.914 14 Shri A S Kulkarni, US Tel-24673965 Email: [email protected] Room No.1021 15 Shri Phool Kanwar, ASO Tel-24197979 Email: [email protected] Room No.1037 OE-I Section 18 Shri Rajesh Sharma, Deputy Secretary Tel-24197909 Email: [email protected] Room No.909 19 Shri Ashok Kumar, US OE-IA Tel-24675142 Email: [email protected] Room No.1022 20 Shri Bijender Singh, SO OE-IA Tel-24197930 Email: [email protected] Room No.7930 21 Shri Varun Yadan, ASO OE-IA -Do- 22 Shri Bikash Ranjan Mahato, US-IB Tel-24197921 Email: [email protected] Room No.921 23 Shri Mukesh Sinha, ASO OE-IB Tel-24197923 Email: [email protected] Room No.923 Consultant 24 Shri Gyan Singh, Consultant OE Division Tel-24197977 Email:[email protected] Room No.1008 *** PROTECTORS OF EMIGRANTS S. -

August Monthly One Liners
August Monthly Current Affairs Business International National Affairs Affairs Awards Important Days Banking Sports Ranking Obituary Sports -CA Funsta LIWIN CA’S FUNSTA Content Title Page Number Important Days 3 Sports 4 Obituaries 7 Ranking 11 Awards 14 Banking And Finance 18 Business And Economy 22 Acquistion And Mergers 23 Books and Authors 24 Appointments 25 International Affairs 34 National Affairs 38 2 LIWIN CA’S FUNSTA IMPORTANT DAYS Date Day Theme August 1 World Lung Cancer Day August 1 – 7 World Breastfeeding Week August 3 Sanskrit Diwas August 6. Hiroshima Day August 7 National Handloom Day August 9 Nagasaki Day August 9 World Tribal Day COVID-19 and Indigenous Peoples' resilience August 10 World Biofuel day Biofuels towards Atmanirbhar Bharat. August 12 International Youth Day August 12 World Elephant Day August 19 World Photography Day August 19 World Humanitarian Day August 20 Sadbhavana Divas August 20 Indian Akshay Urja Day or Renewable Energy Day August 21 World Senior Citizen day August 22 Earth Overshoot Day August 24-28 World Water Week Water and Climate Change : Accelerating Action August 29 National Sports Day 3 LIWIN CA’S FUNSTA Sports Retirement 1. Iker Casillas - announced retirement - Spain’s World Cup-winning captain and goalkeeper 2. Laura Marsh - Former England all-rounder - retirement from all forms of cricket. 3. DN Pathirana and KRP Doolwala – cricket umpires - Sri Lanka - announced Retirement 4. MS Dhoni (Thala) + Suresh Raina = Announces Retirement from International Cricket 5. John Barclay - Former Scotland captain - has announced his retirement from rugby union 6.Ayaka Takahashi - Japan’s Olympic doubles champion – Badminton - announces retirement 7. -

Critical Analysis on History of Kannada Cinema *Dr. B. P. Mahesh
Critical Analysis on History of Kannada Cinema *Dr. B. P. Mahesh Chandra Guru Professor, Department of Studies in Communication and Journalism, University of Mysore, Manasagangotri Karnataka India ** Dr. M.S.Sapna *** M. Prabhudev **** Mr. M. Dileep Kumar Abstract Kannada film industry is indeed an extension of Kannada theatre. The early film personalities had been actively involved in Kannada theatre world. The Kannada film industry had to struggle during 1929 – 1934. Early Kannada films had to struggle against western culture. The film theatres were not equipped well to exhibit silent and talkie films to the audience. The Kannada film industry had recovered from certain setbacks after 1941. About 24 films were made in Kannada after independence. In1950s, Kannada film industry had not gained any identity from the point of view of production of commercial and art films. During 1971 – 1980 several art films and new wave films were made in Kannada. About 138 Kannada films were produced during the decade of 1970s. The decade of 1980s witnessed the production of a large number of commercial Kannada films. There were remarkable economic changes and modifications during 1991 – 2000 in the entire world. Most of the Kannada films were commercial films based on the technique of re-make. In the new millennium, Kannada film industry has grown remarkably. About 80 to 100 films were made every year in Kannada. Kannada film industry has carved a niche for itself in the national and international film avenues. www.ijellh.com 599 Kannada film industry has also incorporated advanced film production technologies and strategies in terms of recording, background music, film song, film editing, special effects, DTS, digital development, use of advanced cameras and so on.