Doing Business Differently the Search for a Cure—Why Now?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Glaadawards March 16, 2013 New York New York Marriott Marquis
#glaadawards MARCH 16, 2013 NEW YORK NEW YORK MARRIOTT MARQUIS APRIL 20, 2013 LOS AnGELES JW MARRIOTT LOS AnGELES MAY 11, 2013 SAN FRANCISCO HILTON SAN FRANCISCO - UnION SQUARE CONNECT WITH US CORPORATE PARTNERS PRESIDENT’S LETTer NOMINEE SELECTION PROCESS speCIAL HONOrees NOMINees SUPPORT FROM THE PRESIDENT Welcome to the 24th Annual GLAAD Media Awards. Thank you for joining us to celebrate fair, accurate and inclusive representations of the lesbian, gay, bisexual and transgender (LGBT) community in the media. Tonight, as we recognize outstanding achievements and bold visions, we also take pause to remember the impact of our most powerful tool: our voice. The past year in news, entertainment and online media reminds us that our stories are what continue to drive equality forward. When four states brought marriage equality to the election FROM THE PRESIDENT ballot last year, GLAAD stepped forward to help couples across the nation to share messages of love and commitment that lit the way for landmark victories in Maine, Maryland, Minnesota and Washington. Now, the U.S. Supreme Court will weigh in on whether same- sex couples should receive the same federal protections as straight married couples, and GLAAD is leading the media narrative and reshaping the way Americans view marriage equality. Because of GLAAD’s work, the Boy Scouts of America is closer than ever before to ending its discriminatory ban on gay scouts and leaders. GLAAD is empowering people like Jennifer Tyrrell – an Ohio mom who was ousted as leader of her son’s Cub Scouts pack – to share their stories with top-tier national news outlets, helping Americans understand the harm this ban inflicts on gay youth and families. -

Global Health Funding Cuts in H.R. 1: Projecting the Human Cost Lives at Stake: in February 2011, the U.S
February 2011 The Foundation for AIDS Research ISSUE BRIEF Global Health Funding Cuts in H.R. 1: Projecting the Human Cost Lives at Stake: In February 2011, the U.S. House of Representatives passed a Continuing Resolution (H.R. 1) to fund the federal government The Potential Annual Human Impact of through the rest of the fiscal year. The legislation would cut more Reducing Bilateral Global Health than $100 billion from the President’s FY 2011 budget request Funding to H.R. 1 Levels and represents the largest spending reduction in Congressional history. • Funding for AIDS treatment for 448,866 people would be eliminated, resulting in a halt to treatment Global health programs represent a high-impact expansion and deeper cuts in HIV prevention and other areas in an effort to avoid removing current investment, advancing American security, patients from lifesaving treatment. diplomatic, and humanitarian objectives. • 299,294 orphans and vulnerable children could lose their food, education, and livelihood assistance. U.S. investments in global health account for only one-quarter • 20,000 more infants could be infected with HIV of 1% of the U.S. budget and they save literally millions of each year due to reductions in services to combat lives each year. These global health programs (through the mother-to-child HIV transmission. President’s Global Health Initiative, bilateral AIDS funding for PEPFAR, and the Global Fund to Fight AIDS, Tuberculosis • Nearly 3.9 million fewer people would be treated and Malaria) represent a high-impact investment, advancing for malaria and 2 million fewer insecticide-treated American security, diplomatic, and humanitarian objectives. -

Amfar India to Benefit Amfar, the Foundation for AIDS Research
amfAR India To Benefit amfAR, The Foundation for AIDS Research November 2014 Mumbai, India Event Produced by Andy Boose / AAB Productions A Golden Opportunity amfAR held its inaugural Indian fundraising gala in Mumbai in November 2013. The event was hosted by amfAR Global Fundraising Chairman Sharon Stone, and Bollywood stars Aishwarya Rai Bachchan and Abhishek Bachchan. It was chaired by amfAR Chairman Kenneth Cole, Vikram Chatwal, Anuj Gupta, Rocky Malhotra, and Hilary Swank. Memorable Moments The black-tie event, presented by Dr. Cyrus Poonawalla and held at The Taj Mahal Palace Hotel, featured a cocktail reception, dinner, and an exquisite gold-themed fashion show that showcased three of India’s most important fashion designers -- Rohit Bal, Abu Jani- Sandeep Khosla, and Tarun Tahiliani. The evening concluded with a spirited performance by pop sensation Ke$ha, whose Hilary Swank for amfAR India songs included her hits Animal, We R Who We R, and Tik Tok. Among the Guests Neeta Ambani, Torquhil Campbell, Duke of Argyll, Sonal Chauhan, Venugopal Dhoot, Nargis Fakhri, Sunil Gavaskar, Parmeshwar Godrej, Lisa Haydon, Dimple Kapadia, Tikka Kapurthala, Nandita Mahtani, Rocky The Love Gold Fashion Show for amfAR India Abhishek Bachchan, Sharon Stone, Nita Ambani, and Aishwarya Rai Bachchan for amfAR India Malhotra, Dino Morea, among many others. Kenneth Cole and Sharon Stone for amfAR India Total Media Impressions: 2.4 Billion Total Media Value: $1.6 Million Aishwarya Rai Bachchan for amfAR India The Love Gold Fashion Show for amfAR India A Star-Studded Cast amfAR’s fundraising events are world renowned for their ability to attract a glittering list of top celebrities, entertainment industry elite, and international society. -

Columbia Global Centers Amman 2015 Annual
“Rather than observing world events from a distance, our students and faculty who A Message from Lee C. Bollinger travel to the global centers With the opening of global centers in Amman and Beijing in 2009, Columbia began an initiative that has become central to our understanding of what it means to be a global university. Amman and Beijing have been joined by six additional Columbia Global Centers, producing a network across four continents that is bringing together great find themselves immersed minds from diverse backgrounds to meet the challenges of our time. in those events and learning The potential of the Columbia Global Centers to provide the intellectual leadership for addressing their regions’ daunting challenges was apparent from the outset. With each passing year, more and more of that potential is being realized, and the results have been gratifying and at times inspiring. The pages of this report set forth those in unique ways from the achievements. All who know the Amman Center are well aware that this growth would not have occurred without the steadfast support of so many. Professor Safwan Masri, Columbia’s Executive Vice President for Global Centers experience.” and Global Development, deserves recognition for his role, as well. At the Columbia Global Centers, Columbia students and faculty are encountering some of the leading figures from Lee C. Bollinger every corner of the globe: innovative scholars, former heads of state, government officials, policy makers, and leaders of industry. This past year, each center hosted a rich variety of programs and events: from workshops assessing the President, Columbia University impact of the refugee crisis on global public health in Amman, to lectures on immigration and African-American cinema in Rio de Janeiro, to panels on women’s rights in Beijing and many more. -

Antiretroviral Drug-Related Liver Mortality Among HIV-Positive
HIV/AIDS MAJOR ARTICLE Antiretroviral Drug-Related Liver Mortality Among HIV-Positive Persons in the Absence of Hepatitis B or C Virus Coinfection: The Data Collection on Adverse Events of Anti-HIV Drugs Study Helen Kovari,1 Caroline A. Sabin,2 Bruno Ledergerber,1 Lene Ryom,3 Signe W. Worm,3 Colette Smith,2 Andrew Phillips,2 Peter Reiss,4 Eric Fontas,5 Kathy Petoumenos,6 Stéphane De Wit,7 Philippe Morlat,8 Jens D. Lundgren,3 and Rainer Weber1 1Division of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich, University of Zurich, Switzerland; 2Research Department of Infection and Population Health, Division of Population Health, Royal Free and University College, London, United Kingdom; 3Copenhagen HIV Programme, University of Copenhagen, Denmark; 4HIV Monitoring Foundation, Academic Medical Center, Amsterdam, The Netherlands; 5Département de Santé Publique, Centre Hospitalier Universitaire, Nice, France; 6National Centre in HIV Epidemiology and Clinical Research, University of New South Wales, Sydney, Australia; 7Department of Infectious Diseases, St Pierre University Hospital, Brussels, Belgium; and 8Service de Medicine Interne et Maladies Infectieuses, Hopital Saint-André, CHU de Bordeaux, France Background. Liver diseases are the leading causes of death in human immunodeficiency virus (HIV)–positive persons since the widespread use of combination antiretroviral treatment (cART). Most of these deaths are due to hepatitis C (HCV) or B (HBV) virus coinfections. Little is known about other causes. Prolonged exposure to some antiretroviral drugs might increase hepatic mortality. Methods. All patients in the Data Collection on Adverse Events of Anti-HIV Drugs study without HCV or HBV coinfection were prospectively followed from date of entry until death or last follow-up. -

CD4:CD8 Ratio Comparison Between Cohorts of HIV-Positive Asians and Caucasians Upon Commencement of Antiretroviral Therapy
HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Antivir Manuscript Author Ther. Author manuscript; Manuscript Author available in PMC 2018 May 03. Published in final edited form as: Antivir Ther. 2017 ; 22(8): 659–668. doi:10.3851/IMP3155. CD4:CD8 ratio comparison between cohorts of HIV-positive Asians and Caucasians upon commencement of antiretroviral therapy Kathy Petoumenos1, Jun Yong Choi2, Jennifer Hoy3, Sasisopin Kiertiburanakul4, Oon Tek Ng5, Mark Boyd6, Reena Rajasuriar7,8, and Matthew Lawon1 on behalf of the Australian HIV Observational Database and the TreatAsia HIV Observational Database 1The Kirby Institute, UNSW Australia, UNSW Sydney NSW 2052, Australia 2Division of Infectious Diseases, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, South Korea 3Department of Infectious Diseases, The Alfred Hospital and Monash University, Melbourne, Australia 4Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand 5Department of Infectious Disease and Communicable Disease Centre, Tan Tock Seng Hospital, Singapore, Singapore 6University of Adelaide, Adelaide, South Australia 5005, Australia 7Centre of Excellence for Research in AIDS (CERiA), University of Malaya, Kuala Lumpur, Malaysia 8Department of Pharmacy, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia Abstract Background—In the era of effective antiretroviral treatment (ART) CD4:CD8 ratio is proposed as a potential marker for HIV-positive (HIV+) patients at increased risk for non-AIDS comorbidities. The current study aims to compare CD4:CD8 ratio between Asian and Caucasian HIV+ patients. Methods—HIV+ patients from the Australian HIV Observational Database (AHOD) and the TREAT Asia HIV Observational Database (TAHOD) meeting specific criteria were included. In these analyses Asian and Caucasian status were defined by cohort. -

ISSUE BRIEF AIDS Research: Broad Health and Economic Benefits
The Foundation for AIDS Research July 2011 ISSUE BRIEF AIDS Research: Broad Health and Economic Benefits Investments in health research at the National Institutes of Health (NIH) have paid enormous dividends in the health and well-being of people in the U.S. and around the world. HIV and AIDS research supported by NIH has yielded important recent advances and holds great promise for significantly reducing HIV infection rates and providing more effective treatments for people living with HIV/AIDS. Yet years of erratic funding levels for NIH and declining purchasing power have undermined our nation’s leadership in health research and our scientists’ ability to take advantage of the expanding opportunities to advance healthcare. The race to find better treatments and cures for cancer, heart Funding for AIDS and overall NIH health research, FY2003–FY2011, in current and inflation-adjusted dollars. The red lines show funding in disease, AIDS, and other diseases—and to control global constant 2003 dollars and show the progressive loss of purchasing power epidemics such as AIDS, tuberculosis, and malaria—depends for AIDS and overall health research funding. (Source: Office of AIDS on robust long-term investment in health research at NIH. Research, National Institutes of Health) Extraordinary Accomplishments in AIDS Research The U.S. has been the global leader in research to prevent and 1982: Scientists name a new disease, acquired treat HIV/AIDS and related serious health conditions. Over the immune deficiency syndrome (AIDS), a year after its past decades, the comprehensive AIDS research program at the discovery. NIH has led to scientific advances that have saved the lives of millions of people with HIV/AIDS and prevented millions more from 1984: American and French scientists confirm that becoming infected. -

Financial Statements Together with Report of Independent Certified Public Accountants
Financial Statements Together with Report of Independent Certified Public Accountants THE FOUNDATION FOR AIDS RESEARCH (formerly known as The American Foundation for AIDS Research) September 30, 2019 and 2018 Contents Page Report of Independent Certified Public Accountants 3 Financial Statements Statements of Financial Position as of September 30, 2019 and 2018 5 Statements of Activities for the years ended September 30, 2019 and 2018 6 Statement of Functional Expenses for the year ended September 30, 2019 7 Statement of Functional Expenses for the year ended September 30, 2018 8 Statements of Cash Flows for the years ended September 30, 2019 and 2018 9 Notes to the Financial Statements 10 GRANT THORNTON LLP REPORT OF INDEPENDENT CERTIFIED PUBLIC ACCOUNTANTS 757 Third Ave., 9th Floor New York, NY 10017-2013 D 212 599 0100 F 212 370 4520 S linkd.in/grantthorntonus To the Board of Trustees of twitter.com/grantthorntonus The Foundation for AIDS Research: We have audited the accompanying financial statements of The Foundation for AIDS Research (a New York not-for-profit corporation, also known as “amfAR”), which comprise the statements of financial position as of September 30, 2019 and 2018, and the related statements of activities, functional expenses and cash flows for the years then ended, and the related notes to the financial statements. Management’s responsibility for the financial statements Management is responsible for the preparation and fair presentation of these financial statements in accordance with accounting principles generally accepted in the United States of America; this includes the design, implementation, and maintenance of internal control relevant to the preparation and fair presentation of financial statements that are free from material misstatement, whether due to fraud or error. -

Approaching a Cure for AIDS Defining an AIDS Cure
Approaching a Cure for AIDS Jeffrey Laurence, M.D. Senior Scientific Consultant for Programs, amfAR Professor of Medicine, Weill Cornell Medical College www.amfar.org www.amfar.org Defining an AIDS Cure Definition: “Treat a disease such that the patient no longer needs to continue treatment, as opposed to suppression or management of the disease as is presently required in diabetes, rheumatoid arthritis, and HIV.” * Specific: Functional vs. sterilizing cures *Fauci A. XVIIth International AIDS Conference, www.amfar.org Mexico City, Aug 3-8, 2008, Abst WESS0101 1 We want to cure HIV. “Viruses can’t be cured.” “Viruses can’t be cured…so far” •In 2009, NIAID spent $41million on HIV cure research, 3% of budget •(~$600million spent on vaccine research) •amfAR filling the gaping hole www.amfar.org A Cure for AIDS •AS Fauci: “We need a cure for AIDS.”* •ART is a life-long commitment •Current ART, and likely every newly developed antiretroviral drug, will have long-term, potentially life-limiting, adverse effects •2.7 million people were newly HIV infected in 2007, with ART reaching only 30% of those who needed it •It is unlikely that we can treat our way out of this pandemic: For every person started on ART, 2-3 are newly infected *Fauci A. XVIIth International AIDS Conference, www.amfar.org Mexico City, Aug 3-8, 2008, Abst WESS0101 2 “The U.S. spends about $20 billion every year on AIDS (both for programs in the U.S. and globally). The money is keeping millions of people alive around the world and preventing millions of infections. -
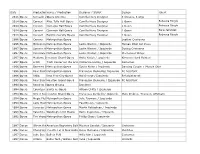
Date Production Name Venue / Production Designer / Stylist Design
Date ProductionVenue Name / Production Designer / Stylist Design Talent 2015 Opera Komachi atOpera Sekidrera America Camilla Huey Designer 3 dresses, 3 wigs 2014 Opera Concert Alice Tully Hall Opera Camilla Huey Designer 1 Gown Rebecca Ringle 2014 Opera Concert Carnegie Hall Opera Camilla Huey Designer 1 Gown Rebecca Ringle 2014 Opera Concert Carnegie Hall Opera Camilla Huey Designer 1 Gown Sara Jakubiak 2013 Opera Concert Bard University Opera Camilla Huey Designer 1 Gown Rebecca Ringle 1996 Opera Carmen Metropolitan Opera Leather Costumes 1996 Opera Midsummer'sMetropolitan Night's Dream Opera Leslie Weston / Izquierdo Human Pillar Set Piece 1997 Opera Samson & MetropolitanDelilah Opera Leslie Weston / Izquierdo Dyeing Costumes 1997 Opera Cerentola Metropolitan Opera Leslie Weston / Izquierdo Mechanical Wings 1997 Opera Madame ButterflyHouston Grand Opera Anita Yavich / Izquierdo Kimonos Hand Painted 1997 Opera Lillith Tisch Center for the Arts OperaCatherine Heraty / Izquierdo Costumes 1996 Opera Bartered BrideMetropolitan Opera Sylvia Nolan / Izquierdo Dancing Couple + Muscle Shirt 1996 Opera Four SaintsMetropolitan In Three Acts Opera Francesco Clemente/ Izquierdo FC Asssitant 1996 Opera Atilla New York City Opera Hal George / Izquierdo Refurbishment 1995 Opera Four SaintsHouston In Three Grand Acts Opera Francesco Clemente / Izquierdo FC Assistant 1994 Opera Requiem VariationsOpera Omaha Izquierdo 1994 Opera Countess MaritzaSanta Fe Opera Allison Chitty / Izquierdo 1994 Opera Street SceneHouston Grand Opera Francesca Zambello/ Izquierdo -

US Philanthropic Support to HIV-AIDS in 2007
07 FUNDERS U.S. Philanthropic Support to Address HIV/AIDS in 2007 CONCERNED ABOUT AIDS Acknowledgements FCAA wishes to thank UNAIDS for its very generous funding and in-kind support of this publication. The data, findings, and conclusions presented in this report are those of FCAA alone and do not necessarily reflect the perspectives or the opinions of UNAIDS, or any other funder. FCAA also gratefully acknowledges the efforts of the following people in ensuring the quality and comprehensiveness of this report: l FCAA Board members Anu Gupta of Johnson & Johnson, Mark Ishaug of AIDS Foundation of Chicago, and Jennifer Kates of The Henry J. Kaiser Family Foundation l Jose Antonio Izazola of UNAIDS, and Astrid Bonfield of the European HIV/AIDS Funders Group (EFG) and The Diana, Princess of Wales Memorial Fund l Jacob Gayle of the Ford Foundation FCAA thanks all the philanthropic entities who responded to the 2007 FCAA resource tracking survey that provided the bulk of information for this publication. Thanks also to Foundation Center and Foundation Search for use of their data to supplement this report. Finally, FCAA thanks all the funding institutions that supported us with a membership contribution in 2008 — our work to build community in HIV/AIDS philanthropy would not be possible without your support. U.S. Philanthropic Support to Address HIV/AIDS in 2007 Table of Executive Summary Contents 2 Executive Summary Total HIV/AIDS-related philanthropy among U.S.-based funders increased between 2006 and 2007, continuing the trend of the last several years. Estimated HIV/AIDS-related funding disbursements by 4 About FCAA and U.S.-based philanthropies grew from $504 million in 2006 to $555 million in 2007, a 10% increase. -
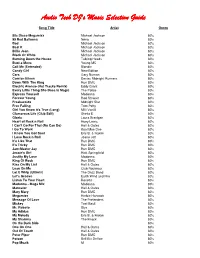
MP3 Database.Wdb
Audio Tech DJ's Music Selection Guide Song Title Artist Genre 80s Disco Megamixx Michael Jackson 80's 99 Red Balloons Nena 80's Bad Michael Jackson 80's Beat It Michael Jackson 80's Billie Jean Michael Jackson 80's Black Or White Michael Jackson 80's Burning Down the House Talking Heads 80's Bust a Move Young MC 80's Call Me (Extended) Blondie 80's Candy Girl New Edition 80's Cars Gary Numan 80's Com'on Eileen Dexies Midnight Runners 80's Down With The King Run DMC 80's Electric Avenue (Hot Tracks Remix) Eddy Grant 80's Every Little Thing She Does Is Magic The Police 80's Express Yourself Madonna 80's Forever Young Rod Stewart 80's Freakazoids Midnight Star 80's Free Falling Tom Petty 80's Girl You Know it's True (Long) Milli Vanilli 80's Glamorous Life (Club Edit) Sheila E 80's Gloria Laura Branigan 80's Heart of Rock n Roll Huey Lewis 80's I Can't Go For That (No Can Do) Hall & Oates 80's I Go To Work Kool Moe Dee 80's I Know You Got Soul Eric B. & Rakim 80's I Love Rock n Roll Joane Jett 80's It's Like That Run DMC 80's It's Tricky Run DMC 80's Jam-Master Jay Run DMC 80's Jessie's Girl Rick Springfield 80's Justify My Love Madonna 80's King Of Rock Run DMC 80's Kiss On My List Hall & Oates 80's Lean On Me Club Nouveau 80's Let It Whip (Ultimix) The Dazz Band 80's Let's Groove Earth Wind and Fire 80's Listen To Your Heart Roxette 80's Madonna - Mega Mix Madonna 80's Maneater Hall & Oates 80's Mary Mary Run DMC 80's Megamixx Herbie Hancock 80's Message Of Love The Pretenders 80's Mickey Toni Basil 80's Mr.