Theranostics Deficiency of ATP6V1H Causes Bone Loss by Inhibiting
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Stelios Pavlidis3, Matthew Loza3, Fred Baribaud3, Anthony
Supplementary Data Th2 and non-Th2 molecular phenotypes of asthma using sputum transcriptomics in UBIOPRED Chih-Hsi Scott Kuo1.2, Stelios Pavlidis3, Matthew Loza3, Fred Baribaud3, Anthony Rowe3, Iaonnis Pandis2, Ana Sousa4, Julie Corfield5, Ratko Djukanovic6, Rene 7 7 8 2 1† Lutter , Peter J. Sterk , Charles Auffray , Yike Guo , Ian M. Adcock & Kian Fan 1†* # Chung on behalf of the U-BIOPRED consortium project team 1Airways Disease, National Heart & Lung Institute, Imperial College London, & Biomedical Research Unit, Biomedical Research Unit, Royal Brompton & Harefield NHS Trust, London, United Kingdom; 2Department of Computing & Data Science Institute, Imperial College London, United Kingdom; 3Janssen Research and Development, High Wycombe, Buckinghamshire, United Kingdom; 4Respiratory Therapeutic Unit, GSK, Stockley Park, United Kingdom; 5AstraZeneca R&D Molndal, Sweden and Areteva R&D, Nottingham, United Kingdom; 6Faculty of Medicine, Southampton University, Southampton, United Kingdom; 7Faculty of Medicine, University of Amsterdam, Amsterdam, Netherlands; 8European Institute for Systems Biology and Medicine, CNRS-ENS-UCBL, Université de Lyon, France. †Contributed equally #Consortium project team members are listed under Supplementary 1 Materials *To whom correspondence should be addressed: [email protected] 2 List of the U-BIOPRED Consortium project team members Uruj Hoda & Christos Rossios, Airways Disease, National Heart & Lung Institute, Imperial College London, UK & Biomedical Research Unit, Biomedical Research Unit, Royal -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

PROTEOMIC ANALYSIS of HUMAN URINARY EXOSOMES. Patricia
ABSTRACT Title of Document: PROTEOMIC ANALYSIS OF HUMAN URINARY EXOSOMES. Patricia Amalia Gonzales Mancilla, Ph.D., 2009 Directed By: Associate Professor Nam Sun Wang, Department of Chemical and Biomolecular Engineering Exosomes originate as the internal vesicles of multivesicular bodies (MVBs) in cells. These small vesicles (40-100 nm) have been shown to be secreted by most cell types throughout the body. In the kidney, urinary exosomes are released to the urine by fusion of the outer membrane of the MVBs with the apical plasma membrane of renal tubular epithelia. Exosomes contain apical membrane and cytosolic proteins and can be isolated using differential centrifugation. The analysis of urinary exosomes provides a non- invasive means of acquiring information about the physiological or pathophysiological state of renal cells. The overall objective of this research was to develop methods and knowledge infrastructure for urinary proteomics. We proposed to conduct a proteomic analysis of human urinary exosomes. The first objective was to profile the proteome of human urinary exosomes using liquid chromatography-tandem spectrometry (LC- MS/MS) and specialized software for identification of peptide sequences from fragmentation spectra. We unambiguously identified 1132 proteins. In addition, the phosphoproteome of human urinary exosomes was profiled using the neutral loss scanning acquisition mode of LC-MS/MS. The phosphoproteomic profiling identified 19 phosphorylation sites corresponding to 14 phosphoproteins. The second objective was to analyze urinary exosomes samples isolated from patients with genetic mutations. Polyclonal antibodies were generated to recognize epitopes on the gene products of these genetic mutations, NKCC2 and MRP4. The potential usefulness of urinary exosome analysis was demonstrated using the well-defined renal tubulopathy, Bartter syndrome type I and using the single nucleotide polymorphism in the ABCC4 gene. -

Zebrafish Mutants Calamity and Catastrophe Define Critical Pathways of Gene–Nutrient Interactions in Developmental Copper Metabolism Erik C
Washington University School of Medicine Digital Commons@Becker Open Access Publications 2008 Zebrafish mutants calamity and catastrophe define critical pathways of gene–nutrient interactions in developmental copper metabolism Erik C. Madsen Washington University School of Medicine in St. Louis Jonathan D. Gitlin Washington University School of Medicine in St. Louis Follow this and additional works at: https://digitalcommons.wustl.edu/open_access_pubs Part of the Medicine and Health Sciences Commons Recommended Citation Madsen, Erik C. and Gitlin, Jonathan D., ,"Zebrafish mutants calamity and catastrophe define critical pathways of gene–nutrient interactions in developmental copper metabolism." PLoS Genetics.,. e1000261. (2008). https://digitalcommons.wustl.edu/open_access_pubs/892 This Open Access Publication is brought to you for free and open access by Digital Commons@Becker. It has been accepted for inclusion in Open Access Publications by an authorized administrator of Digital Commons@Becker. For more information, please contact [email protected]. Zebrafish Mutants calamity and catastrophe Define Critical Pathways of Gene–Nutrient Interactions in Developmental Copper Metabolism Erik C. Madsen, Jonathan D. Gitlin¤* Edward Mallinckrodt Department of Pediatrics, Washington University School of Medicine, St. Louis, Missouri, United States of America Abstract Nutrient availability is an important environmental variable during development that has significant effects on the metabolism, health, and viability of an organism. To understand these interactions for the nutrient copper, we used a chemical genetic screen for zebrafish mutants sensitive to developmental copper deficiency. In this screen, we isolated two mutants that define subtleties of copper metabolism. The first contains a viable hypomorphic allele of atp7a and results in a loss of pigmentation when exposed to mild nutritional copper deficiency. -

Identification of Potential Key Genes and Pathway Linked with Sporadic Creutzfeldt-Jakob Disease Based on Integrated Bioinformatics Analyses
medRxiv preprint doi: https://doi.org/10.1101/2020.12.21.20248688; this version posted December 24, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. All rights reserved. No reuse allowed without permission. Identification of potential key genes and pathway linked with sporadic Creutzfeldt-Jakob disease based on integrated bioinformatics analyses Basavaraj Vastrad1, Chanabasayya Vastrad*2 , Iranna Kotturshetti 1. Department of Biochemistry, Basaveshwar College of Pharmacy, Gadag, Karnataka 582103, India. 2. Biostatistics and Bioinformatics, Chanabasava Nilaya, Bharthinagar, Dharwad 580001, Karanataka, India. 3. Department of Ayurveda, Rajiv Gandhi Education Society`s Ayurvedic Medical College, Ron, Karnataka 562209, India. * Chanabasayya Vastrad [email protected] Ph: +919480073398 Chanabasava Nilaya, Bharthinagar, Dharwad 580001 , Karanataka, India NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice. medRxiv preprint doi: https://doi.org/10.1101/2020.12.21.20248688; this version posted December 24, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. All rights reserved. No reuse allowed without permission. Abstract Sporadic Creutzfeldt-Jakob disease (sCJD) is neurodegenerative disease also called prion disease linked with poor prognosis. The aim of the current study was to illuminate the underlying molecular mechanisms of sCJD. The mRNA microarray dataset GSE124571 was downloaded from the Gene Expression Omnibus database. Differentially expressed genes (DEGs) were screened. -

Molecular Mechanism of Embryo Transport and Embryo
MOLECULAR MECHANISM OF EMBRYO TRANSPORT AND EMBRYO IMPLANTATION IN MICE By SHUO XIAO (Under the Direction of Xiaoqin Ye) ABSTRACT Embryo transport and embryo implantation are essential events in mammalian reproduction during pregnancy. This dissertation was conducted to investigate the effect of bisphenol A (BPA) on early pregnancy and the molecular mechanism (s) of embryo transport and embryo implantation. Timed pregnant female mice were treated subcutaneously with 0, 0.025, 0.5, 10, 40, and 100 mg/kg/day BPA from gestation day 0.5 (D0.5, mating night as D0) to D3.5. High dose of preimplantation BPA exposure resulted in delayed embryo transport and preimplantation embryo development, and delayed/failed embryo implantation, indicating the adverse effect of BPA on early pregnancy. Preimplantation 17β-estradiol (E2) exposure at 1 and 10 µg/kg/day from D0.5 to D2.5 delayed embryo transport in the ampulla-isthmus junction of oviduct in mice, which is associated with the oviductal epithelium hyperplasia. Microarray analysis revealed 53 differentially expressed genes in the oviduct upon 10 µg/kg/day E2 treatment, which may have potential function in embryo transport regulation. Embryo implantation is a process that the receptive uterus accepts an embryo to implant into the uterine wall. Microarray analysis of the preimplantation D3.5 and postimplantation D4.5 uterine luminal epithelium (LE) identified 627 differentially expressed genes and 21 significantly changed signaling pathways upon embryo implantation. 12 of these genes were newly characterized and showed spatiotemporal expression patterns in the mouse periimplantation uterine LE. The most upregulated gene in the D4.5 LE Atp6v0d2 (34.7x) is a subunit of vacuolar-type H+-ATPase (V-ATPase) that regulates the cell acidification through ATP hydrolysis and proton translocation. -

ELMO1 Signaling Is a Promoter of Osteoclast Function and Bone Loss ✉ Sanja Arandjelovic 1 , Justin S
ARTICLE https://doi.org/10.1038/s41467-021-25239-6 OPEN ELMO1 signaling is a promoter of osteoclast function and bone loss ✉ Sanja Arandjelovic 1 , Justin S. A. Perry 1, Ming Zhou 2, Adam Ceroi3, Igor Smirnov4, Scott F. Walk1, Laura S. Shankman1, Isabelle Cambré3, Suna Onengut-Gumuscu 5, Dirk Elewaut 3, Thomas P. Conrads 2 & ✉ Kodi S. Ravichandran 1,3 Osteoporosis affects millions worldwide and is often caused by osteoclast induced bone loss. 1234567890():,; Here, we identify the cytoplasmic protein ELMO1 as an important ‘signaling node’ in osteoclasts. We note that ELMO1 SNPs associate with bone abnormalities in humans, and that ELMO1 deletion in mice reduces bone loss in four in vivo models: osteoprotegerin deficiency, ovariectomy, and two types of inflammatory arthritis. Our transcriptomic analyses coupled with CRISPR/Cas9 genetic deletion identify Elmo1 associated regulators of osteoclast function, including cathepsin G and myeloperoxidase. Further, we define the ‘ELMO1 inter- actome’ in osteoclasts via proteomics and reveal proteins required for bone degradation. ELMO1 also contributes to osteoclast sealing zone on bone-like surfaces and distribution of osteoclast-specific proteases. Finally, a 3D structure-based ELMO1 inhibitory peptide reduces bone resorption in wild type osteoclasts. Collectively, we identify ELMO1 as a signaling hub that regulates osteoclast function and bone loss, with relevance to osteoporosis and arthritis. 1 Center for Cell Clearance, Department of Microbiology, Immunology, and Cancer Biology and Carter Immunology Center, University of Virginia, Charlottesville, VA, USA. 2 Inova Schar Cancer Institute, Inova Center for Personalized Health, Fairfax, VA, USA. 3 Inflammation Research Centre, VIB, and the Department of Biomedical Molecular Biology, Ghent Univeristy, Ghent, Belgium. -
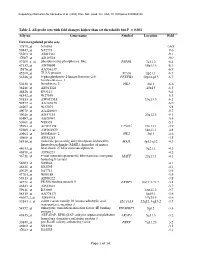
Table 2. All Probe Sets with Fold Changes Higher Than Set Thresholds but P > 0.001 Affy No
Supporting information for Sanoudou et al. (2003) Proc. Natl. Acad. Sci. USA, 10.1073/pnas.0330960100 Table 2. All probe sets with fold changes higher than set thresholds but P > 0.001 Affy no. Gene name Symbol Location Fold Down-regulated probe sets 47879_at N46863 -16.9 59447_at N52773 -9.6 55503_at AI085361 -9.1 47087_at AI310524 -9.0 37209_g_at phosphoserine phosphatase-like PSPHL 7q11.2 -8.4 47137_at AI479899 19p13.3 -8.1 45876_at AA536137 -6.9 45260_at TU3A protein TU3A 3p21.1 -6.7 56246_at 6-phosphofructo-2-kinase/fructose-2,6- PFKFB3 10p14-p15 -6.7 bisphosphatase 3 50230_at hexokinase 2 HK2 2p13 -6.6 38248_at AB011124 20p13 -6.5 44426_at R93141 -6.4 48542_at W27559 -6.3 53813_at AW051518 13q13.3 -6.1 59577_at AA243670 -6.0 46907_at W37075 -5.8 49078_at AA424983 -5.7 49026_at AI357153 20p12.3 -5.4 53487_at AI670947 -5.4 50161_at N39328 -5.1 35994_at AC002398 F25965 19q13.1 -4.9 52285_f_at AW002970 18p11.1 -4.8 40964_at hexokinase 2 HK2 2p13 -4.6 49806_at AI932283 -4.5 58918_at molecule possessing ankyrin repeats induced by MAIL 3p12-q12 -4.3 lipopolysaccharide (MAIL), homolog of mouse 44633_at heat shock 27 kDa associated protein 3q21.1 -4.3 46858_at AI796221 -4.2 36711_at v-maf musculoaponeurotic fibrosarcoma oncogene MAFF 22q13.1 -4.1 homolog F (avian) 54683_at N49844 -4.1 46621_at N32595 -4.1 49629_at N47713 -3.9 47703_at W89189 -3.9 59313_at AI598222 -3.8 34721_at FK506 binding protein 5 FKBP5 6p21.3-21.2 -3.8 46843_at AI632621 -3.7 59611_at R53069 16p11.2 -3.7 58315_at AA778171 3p25.1 -3.6 46607_f_at AI885018 17q25.3 -3.6 33143_s_at solute carrier family 16 (monocarboxylic acid SLC16A3 22q12.3-q13.2 -3.5 transporters), member 3 54152_at eukaryotic translation initiation factor 4E binding EIF4EBP1 8p12 -3.4 protein 1 43935_at ARF-GAP, RHO-GAP, ankyrin repeat and plekstrin ARAP3 5q31.3 -3.2 homology domains-containing protein 3 33849_at pre-B-cell colony-enhancing factor PBEF 7q11.23 -3.2 46902_at N92294 -3.2 47023_at N25555 -3.1 Page 1 of 14 Supporting information for Sanoudou et al. -

Discovery of Molecular Mechanisms Underlying Lysosomal and Mitochondrial Defects in Parkinson’S Disease
Discovery of Molecular Mechanisms Underlying Lysosomal and Mitochondrial Defects In Parkinson’s Disease Brigitte Phillips Supervisor: Associate Professor Antony Cooper A thesis in fulfillment of the requirements for the degree of Doctor of Philosophy St Vincent’s Clinical School, Faculty of Medicine The University of New South Wales & The Garvan Institute of Medical Research April, 2018 THE UNIVERSITY OF NEW SOUTH WALES Thesis/Dissertation Sheet Surname or Family name: Phillips First name: Brigitte Other names/s: Radinovic Abbreviation for degree as given in the University calendar: PhD School: St Vincent’s Clinical School Title: The emerging contributions of the lysosome and mitochondria to Parkinson’s diseases Abstract 350 words maximum Parkinson’s disease (PD) is a common, debilitating neurodegenerative disease yet the causes of cell dysfunction in PD remain unclear. By integrating available patient data with data from an unbiased assessment of proteomic changes in multiple cellular PD models, this study has identified new aspects of mitochondrial and lysosomal dysfunction that likely contribute to PD. Two areas were investigated in detail; the mitochondrial protein CHCHD2 and the V-ATPase complex, which acidifies endolysosomal compartments. CHCHD2 had not been well characterized or associated with PD when identified in this study, however PD-causative variants have since been described and CHCHD2 has recently been proposed to regulate mitochondrial cristae structure and interact with cytochrome c. Data from this thesis extends CHCHD2 dysfunction to sporadic PD patients, where its reduced expression was identified in the brain. In exploring the potential function of CHCHD2, mitochondrial impairment resulted in rapid translational up- regulation of CHCHD2 and its specific accumulation in depolarised mitochondria, suggesting that CHCHD2 plays a targeted role in aiding mitochondrial recovery or quarantining cytochrome c in response to mitochondrial damage. -

Kidney V-Atpase-Rich Cell Proteome Database
A comprehensive list of the proteins that are expressed in V-ATPase-rich cells harvested from the kidneys based on the isolation by enzymatic digestion and fluorescence-activated cell sorting (FACS) from transgenic B1-EGFP mice, which express EGFP under the control of the promoter of the V-ATPase-B1 subunit. In these mice, type A and B intercalated cells and connecting segment principal cells of the kidney express EGFP. The protein identification was performed by LC-MS/MS using an LTQ tandem mass spectrometer (Thermo Fisher Scientific). For questions or comments please contact Sylvie Breton ([email protected]) or Mark A. Knepper ([email protected]). -

Molecular Architecture Underlying Fluid Absorption by the Developing Inner
RESEARCH ARTICLE Molecular architecture underlying fluid absorption by the developing inner ear Keiji Honda1†, Sung Huhn Kim2‡, Michael C Kelly3, Joseph C Burns3§, Laura Constance2, Xiangming Li2#, Fei Zhou2, Michael Hoa4, Matthew W Kelley3, Philine Wangemann2*, Robert J Morell5, Andrew J Griffith1* 1Molecular Biology and Genetics Section, National Institute on Deafness and Other Communication Disorders, National Institutes of Health, Bethesda, United States; 2Anatomy and Physiology Department, Kansas State University, Manhattan, United States; 3Developmental Neuroscience Section, National Institute on Deafness and Other Communication Disorders, National Institutes of Health, Bethesda, United States; 4Auditory Development and Restoration Program, National Institute on *For correspondence: Deafness and Other Communication Disorders, National Institutes of Health, [email protected] (PW); Bethesda, United States; 5Genomics and Computational Biology Core, National [email protected] (AJG) Institute on Deafness and Other Communication Disorders, National Institutes of Present address: Health, Bethesda, United States †Otolaryngology Department, Tsuchiura Kyodo General Hospital, Tsuchiura, Japan; ‡Department of Abstract Mutations of SLC26A4 are a common cause of hearing loss associated with Otorhinolaryngology, Head and enlargement of the endolymphatic sac (EES). Slc26a4 expression in the developing mouse Neck Surgery, Yonsei University College of Medicine, Seoul, endolymphatic sac is required for acquisition of normal inner ear structure and function. Here, we Korea; §Decibel Therapeutics, show that the mouse endolymphatic sac absorbs fluid in an SLC26A4-dependent fashion. Fluid Cambridge, United States; absorption was sensitive to ouabain and gadolinium but insensitive to benzamil, bafilomycin and #Technique R and D-Drug S3226. Single-cell RNA-seq analysis of pre- and postnatal endolymphatic sacs demonstrates two Substance, GlaxoSmithKline types of differentiated cells. -

Robles JTO Supplemental Digital Content 1
Supplementary Materials An Integrated Prognostic Classifier for Stage I Lung Adenocarcinoma based on mRNA, microRNA and DNA Methylation Biomarkers Ana I. Robles1, Eri Arai2, Ewy A. Mathé1, Hirokazu Okayama1, Aaron Schetter1, Derek Brown1, David Petersen3, Elise D. Bowman1, Rintaro Noro1, Judith A. Welsh1, Daniel C. Edelman3, Holly S. Stevenson3, Yonghong Wang3, Naoto Tsuchiya4, Takashi Kohno4, Vidar Skaug5, Steen Mollerup5, Aage Haugen5, Paul S. Meltzer3, Jun Yokota6, Yae Kanai2 and Curtis C. Harris1 Affiliations: 1Laboratory of Human Carcinogenesis, NCI-CCR, National Institutes of Health, Bethesda, MD 20892, USA. 2Division of Molecular Pathology, National Cancer Center Research Institute, Tokyo 104-0045, Japan. 3Genetics Branch, NCI-CCR, National Institutes of Health, Bethesda, MD 20892, USA. 4Division of Genome Biology, National Cancer Center Research Institute, Tokyo 104-0045, Japan. 5Department of Chemical and Biological Working Environment, National Institute of Occupational Health, NO-0033 Oslo, Norway. 6Genomics and Epigenomics of Cancer Prediction Program, Institute of Predictive and Personalized Medicine of Cancer (IMPPC), 08916 Badalona (Barcelona), Spain. List of Supplementary Materials Supplementary Materials and Methods Fig. S1. Hierarchical clustering of based on CpG sites differentially-methylated in Stage I ADC compared to non-tumor adjacent tissues. Fig. S2. Confirmatory pyrosequencing analysis of DNA methylation at the HOXA9 locus in Stage I ADC from a subset of the NCI microarray cohort. 1 Fig. S3. Methylation Beta-values for HOXA9 probe cg26521404 in Stage I ADC samples from Japan. Fig. S4. Kaplan-Meier analysis of HOXA9 promoter methylation in a published cohort of Stage I lung ADC (J Clin Oncol 2013;31(32):4140-7). Fig. S5. Kaplan-Meier analysis of a combined prognostic biomarker in Stage I lung ADC.