Full Article
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Actinorhizal Plants of Kumaun Himalaya and Their Ecological
International Scholars Journals Advances in Agriculture and Agricultural Sciences ISSN 2381-3911 Vol. 6 (7), pp. 001-006, July, 2020. Available online at www.internationalscholarsjournals.org © International Scholars Journals Author(s) retain the copyright of this article. Full Length Research Paper Actinorhizal plants of Kumaun Himalaya and their ecological significance Kiran Bargali Department of Botany, Kumaun University (K.U.) Nainital-263002, Uttarakhand, India. E-mail: [email protected] Accepted 21 June, 2020 Actinorhizal plants are important in having symbiotic association with actinomycete Frankia. Nitrogen (N) fixation by actinorhizal plants is a major source of fixed N in diverse and widespread ecosystems including forests, bogs, swamps, coastal dunes, landslides, glacial deposits, shrublands, prairies and deserts. They play important roles in wild land ecosystem function and are used in land reclamation, range management, forestry, agroforestry and horticulture. In this study, 8 actinorhizal plants of Kumaun Himalayan region have been described. Habit, habitat, distribution and possible ecological significance of these plants are also described. These plants are not only important for the restoration of degraded lands, but also provide good source of timber, fuel wood, fodder, food and medicines, etc. Key words: Actinorhizal, degraded, nitrogen, plantation, reclamation. INTRODUCTION Frankia, a filamentous bacterium (actinomycete), forms In the present study, an attempt has been made to nodule like symbiotic associations with the roots of several identify the actinorhizal plants growing in Kumaun plant species known collectively as "Actinorhizal plant". The Himalayan region. The recent application of plant actinorhizal symbiosis have been reported in numerous nitrogen fixation in ecosystem is a key step to understand plants all over the word except Antartica (Baker and its implications in ecological conservation, and future Schwinterzer, 1990). -

Integrating Local Knowledge with Tree Diversity Analyses to Optimize On
Agroforest Syst (2019) 93:755–770 https://doi.org/10.1007/s10457-017-0172-8 Integrating local knowledge with tree diversity analyses to optimize on-farm tree species composition for ecosystem service delivery in coffee agroforestry systems of Uganda Hannington Bukomeko . Laurence Jassogne . Susan Balaba Tumwebaze . Gerald Eilu . Philippe Vaast Received: 16 December 2016 / Accepted: 5 December 2017 / Published online: 8 December 2017 Ó The Author(s) 2017. This article is an open access publication Abstract Coffee agroforestry systems deliver of importance to their livelihoods (‘Needs rank’) and ecosystem services (ES) critical for rural livelihoods ranked trees according to suitability for providing ES. like food but also disservices that constrain livelihoods Using Bradley Terry modeling, we grouped trees into like fostering coffee-pests. Since such ES are tree- ‘ES groups’ according to suitability for providing based, maximizing ES and limiting constraints different ES and ranked ‘ES groups’ according to tree requires knowledge on optimizing on-farm tree com- diversity (‘Diversity rank’). Tree-suitability for pro- position especially trees adapted to local conditions. viding ES and importance of ES to farmers varied with The study was in three sites along a rainfall gradient in rainfall regime but tree diversity did not match Central Uganda where we: assessed tree diversity in farmers’ needs for ES. We propose the FaD–FaN coffee agroforestry; ranked tree suitability for provid- (matching farm tree diversity to farmers’ needs) ing ES according to farmers’ knowledge; and then approach for optimizing tree species composition with proposed an approach for optimizing on-farm tree respect to tree-suitability for farmers’ priority ES. -

Rhus Coriaria
Rhus coriaria (Elm-Leaved Sumach, Sicilian sumac) " Every Lebanese has eaten the dark red condiment called summac, which is derived from the fruits of this tree and is used in popular foods such as the Mankouche, Kaakeh, and Fattoush.Summac tree is found in every part of the country, growing at almost every altitude. This small tree, composed of single or multiple slender trunks, reaches 2 to 3 meters in height.The Summac tree can grow in poor, rocky soils with little access to water; it is tolerant to drought and grows well along the coast, because it is also resistant to salt." from: Trees of Lebanon, 2014, Salma Nashabe Talhouk, Mariana M. Yazbek, Khaled Sleem, Arbi J. Sarkissian, Mohammad S. Al-Zein, and Sakra Abo Eid Landscape Information French Name: Le Sumac des corroyeurs ﺳﻤﺎﻕ :Arabic Name Plant Type: Shrub Origin: Southern Europe Heat Zones: 4, 5, 6, 7, 8 Hardiness Zones: 8, 9, 10, 11 Uses: Medicinal, Native to Lebanon Size/Shape Growth Rate: Moderate Tree Shape: Spreading Canopy Symmetry: Irregular Canopy Density: Dense Plant Image Canopy Texture: Medium Height at Maturity: 1.5 to 3 m Spread at Maturity: 3 to 5 meters Time to Ultimate Height: 10 to 20 Years Rhus coriaria (Elm-Leaved Sumach, Sicilian sumac) Botanical Description Foliage Leaf Arrangement: Whorled Leaf Venation: Pinnate Leaf Persistance: Deciduous Leaf Type: Odd Pinnately compund Leaf Blade: 5 - 10 cm Leaf Shape: Oval Leaf Margins: Serrate Leaf Textures: Hairy, Fine Leaf Scent: No Fragance Color(growing season): Green Color(changing season): Green Flower -

Report of Rapid Biodiversity Assessments at Cenwanglaoshan Nature Reserve, Northwest Guangxi, China, 1999 and 2002
Report of Rapid Biodiversity Assessments at Cenwanglaoshan Nature Reserve, Northwest Guangxi, China, 1999 and 2002 Kadoorie Farm and Botanic Garden in collaboration with Guangxi Zhuang Autonomous Region Forestry Department Guangxi Forestry Survey and Planning Institute South China Institute of Botany South China Normal University Institute of Zoology, CAS March 2003 South China Forest Biodiversity Survey Report Series: No. 27 (Online Simplified Version) Report of Rapid Biodiversity Assessments at Cenwanglaoshan Nature Reserve, Northwest Guangxi, China, 1999 and 2002 Editors John R. Fellowes, Bosco P.L. Chan, Michael W.N. Lau, Ng Sai-Chit and Gloria L.P. Siu Contributors Kadoorie Farm and Botanic Garden: Gloria L.P. Siu (GS) Bosco P.L. Chan (BC) John R. Fellowes (JRF) Michael W.N. Lau (ML) Lee Kwok Shing (LKS) Ng Sai-Chit (NSC) Graham T. Reels (GTR) Roger C. Kendrick (RCK) Guangxi Zhuang Autonomous Region Forestry Department: Xu Zhihong (XZH) Pun Fulin (PFL) Xiao Ma (XM) Zhu Jindao (ZJD) Guangxi Forestry Survey and Planning Institute (Comprehensive Tan Wei Fu (TWF) Planning Branch): Huang Ziping (HZP) Guangxi Natural History Museum: Mo Yunming (MYM) Zhou Tianfu (ZTF) South China Institute of Botany: Chen Binghui (CBH) Huang Xiangxu (HXX) Wang Ruijiang (WRJ) South China Normal University: Li Zhenchang (LZC) Chen Xianglin (CXL) Institute of Zoology CAS (Beijing): Zhang Guoqing (ZGQ) Chen Deniu (CDN) Nanjing University: Chen Jianshou (CJS) Wang Songjie (WSJ) Xinyang Teachers’ College: Li Hongjing (LHJ) Voluntary specialist: Keith D.P. Wilson (KW) Background The present report details the findings of visits to Northwest Guangxi by members of Kadoorie Farm and Botanic Garden (KFBG) in Hong Kong and their colleagues, as part of KFBG's South China Biodiversity Conservation Programme. -

They Come in Teams
GBE Frankia-Enriched Metagenomes from the Earliest Diverging Symbiotic Frankia Cluster: They Come in Teams Thanh Van Nguyen1, Daniel Wibberg2, Theoden Vigil-Stenman1,FedeBerckx1, Kai Battenberg3, Kirill N. Demchenko4,5, Jochen Blom6, Maria P. Fernandez7, Takashi Yamanaka8, Alison M. Berry3, Jo¨ rn Kalinowski2, Andreas Brachmann9, and Katharina Pawlowski 1,* 1Department of Ecology, Environment and Plant Sciences, Stockholm University, Sweden 2Center for Biotechnology (CeBiTec), Bielefeld University, Germany 3Department of Plant Sciences, University of California, Davis 4Laboratory of Cellular and Molecular Mechanisms of Plant Development, Komarov Botanical Institute, Russian Academy of Sciences, Saint Petersburg, Russia 5Laboratory of Molecular and Cellular Biology, All-Russia Research Institute for Agricultural Microbiology, Saint Petersburg, Russia 6Bioinformatics and Systems Biology, Justus Liebig University, Gießen, Germany 7Ecologie Microbienne, Centre National de la Recherche Scientifique UMR 5557, Universite Lyon I, Villeurbanne Cedex, France 8Forest and Forestry Products Research Institute, Ibaraki, Japan 9Biocenter, Ludwig Maximilians University Munich, Planegg-Martinsried, Germany *Corresponding author: E-mail: [email protected]. Accepted: July 10, 2019 Data deposition: This project has been deposited at EMBL/GenBank/DDBJ under the accession PRJEB19438 - PRJEB19449. Abstract Frankia strains induce the formation of nitrogen-fixing nodules on roots of actinorhizal plants. Phylogenetically, Frankia strains can be grouped in four clusters. The earliest divergent cluster, cluster-2, has a particularly wide host range. The analysis of cluster-2 strains has been hampered by the fact that with two exceptions, they could never be cultured. In this study, 12 Frankia-enriched meta- genomes of Frankia cluster-2 strains or strain assemblages were sequenced based on seven inoculum sources. Sequences obtained via DNA isolated from whole nodules were compared with those of DNA isolated from fractionated preparations enhanced in the Frankia symbiotic structures. -

Phylogenetic Relationships in the Order Cucurbitales and a New Classification of the Gourd Family (Cucurbitaceae)
Schaefer & Renner • Phylogenetic relationships in Cucurbitales TAXON 60 (1) • February 2011: 122–138 TAXONOMY Phylogenetic relationships in the order Cucurbitales and a new classification of the gourd family (Cucurbitaceae) Hanno Schaefer1 & Susanne S. Renner2 1 Harvard University, Department of Organismic and Evolutionary Biology, 22 Divinity Avenue, Cambridge, Massachusetts 02138, U.S.A. 2 University of Munich (LMU), Systematic Botany and Mycology, Menzinger Str. 67, 80638 Munich, Germany Author for correspondence: Hanno Schaefer, [email protected] Abstract We analysed phylogenetic relationships in the order Cucurbitales using 14 DNA regions from the three plant genomes: the mitochondrial nad1 b/c intron and matR gene, the nuclear ribosomal 18S, ITS1-5.8S-ITS2, and 28S genes, and the plastid rbcL, matK, ndhF, atpB, trnL, trnL-trnF, rpl20-rps12, trnS-trnG and trnH-psbA genes, spacers, and introns. The dataset includes 664 ingroup species, representating all but two genera and over 25% of the ca. 2600 species in the order. Maximum likelihood analyses yielded mostly congruent topologies for the datasets from the three genomes. Relationships among the eight families of Cucurbitales were: (Apodanthaceae, Anisophylleaceae, (Cucurbitaceae, ((Coriariaceae, Corynocarpaceae), (Tetramelaceae, (Datiscaceae, Begoniaceae))))). Based on these molecular data and morphological data from the literature, we recircumscribe tribes and genera within Cucurbitaceae and present a more natural classification for this family. Our new system comprises 95 genera in 15 tribes, five of them new: Actinostemmateae, Indofevilleeae, Thladiantheae, Momordiceae, and Siraitieae. Formal naming requires 44 new combinations and two new names in Cucurbitaceae. Keywords Cucurbitoideae; Fevilleoideae; nomenclature; nuclear ribosomal ITS; systematics; tribal classification Supplementary Material Figures S1–S5 are available in the free Electronic Supplement to the online version of this article (http://www.ingentaconnect.com/content/iapt/tax). -

Picrotoxane Sesquiterpene Glycosides and a Coumarin Derivative from Coriaria Nepalensis and Their Neurotrophic Activity
molecules Article Picrotoxane Sesquiterpene Glycosides and a Coumarin Derivative from Coriaria nepalensis and Their Neurotrophic Activity Yuan-Yuan Wang, Jun-Mian Tian, Cheng-Chen Zhang, Bo Luo and Jin-Ming Gao * Shaanxi Key Laboratory of Natural Products & Chemical Biology, College of Science, Northwest A&F University, Yangling 712100, Shaanxi, China; [email protected] (Y.-Y.W.); [email protected] (J.-M.T.); [email protected] (C.-C.Z.); [email protected] (B.L.) * Correspondence: [email protected]; Tel.: +86-29-8709-2335; Fax: +86-29-8709-2226 Academic Editor: Isabel C. F. R. Ferreira Received: 10 September 2016; Accepted: 9 October 2016; Published: 12 October 2016 Abstract: Two picrotoxane sesquiterpene lactone glycosides, nepalactones A (1) and B (2), and one new coumarin, nepalarin (3), were isolated from the root barks of the poisonous plant Coriaria nepalensis. Their structures were elucidated via HRESIMS and 1D and 2D NMR spectroscopic analyses, and further verified via transformation methods. In addition, compounds 1–3 and five semisynthetic congeners (1a–e) were assayed for the activity to induce neurite outgrowth in rat pheochromocytoma (PC12) cells. As a result, nepalactone A derivative 1c and nepalarin (3) significantly enhanced nerve growth factor (NGF)-mediated neurite outgrowth in PC12 cells. Keywords: Coriaria nepalensis; Coriariaceae; natural products; picrotoxane; sesquiterpene; neurite outgrowth-potentiating activity; neurotrophic activity 1. Introduction Coriaria nepalensis Wall (Coriaria sinica Maxim) (Coriariaceae) is a Chinese medicine herb mainly distributed in the southwest of China. Traditionally, this herb is used to treat numbness, toothache, traumatic injury, and acute conjunctivitis [1]. Sesquiterpene lactones are the characteristic bioactive constituents from family Coriariaceae [2–9]. -
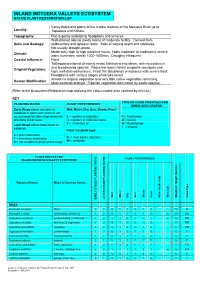
Inland Motueka Valleys Plant Lists
INLAND MOTUEKA VALLEYS ECOSYSTEM NATIVE PLANT RESTORATION LIST Valley floors and plains of the middle reaches of the Motueka River up to Locality: Tapawera and Kohatu. Topography: Flat to gently undulating floodplains and terraces. Well-drained alluvial sandy loams of moderate fertility. Derived from Soils and Geology: sedimentary and igneous rocks. Soils of varying depth and stoniness. Not usually drought-prone. Moderately high to high sunshine hours; frosts moderate to moderately severe; Climate: warm summers; rainfall 1200-1600mm. Droughts infrequent. Coastal influence: None Tall podocarp forest of mainly mataī, kāhikatea and tōtara, with mixed beech and broadleaved species. Red-silver beech forest on gentle toeslopes and Original Vegetation: high, well-drained terraces. Frost flat shrublands on terraces with severe frost. Floodplains with various stages of kānuka forest. Almost no original vegetation and very little native vegetation remaining. Human Modification: Most wetlands drained. Riparian vegetation dominated by exotic species. [Refer to the Ecosystem Restoration map showing the colour-coded area covered by this list.] KEY TYPE OF FOOD PROVIDED FOR PLANTING RATIO PLANT PREFERENCES BIRDS AND LIZARDS Early Stage plants are able to Wet, Moist, Dry, Sun, Shade, Frost establish in open sites and can act as a nursery for later stage plants by 1 = prefers or tolerates F = Fruit/seeds providing initial cover. ½ = prefers or tolerates some N = Nectar 0 = intolerant of B = Buds/foliage Later Stage plants need cover to I = Insects establish. -

Early Evolution of Coriariaceae (Cucurbitales) in Light of a New Early Campanian (Ca
TAXON 69 (1) • February 2020: 87–99 Renner & al. • Evolution of Coriariaceae SYSTEMATICS AND PHYLOGENY Early evolution of Coriariaceae (Cucurbitales) in light of a new early Campanian (ca. 82 Mya) pollen record from Antarctica Susanne S. Renner,1 Viviana D. Barreda,2 María Cristina Tellería,3 Luis Palazzesi2 & Tanja M. Schuster1 1 Systematic Botany and Mycology, Ludwig Maximilian University of Munich (LMU), Menzinger-Straße 67, 80638 Munich, Germany 2 Museo Argentino de Ciencias Naturales, Av. Ángel Gallardo 470, C1407DJR Buenos Aires, Argentina 3 Laboratorio de Sistemática y Biología Evolutiva, Museo de La Plata, La Plata, B1900FWA, Argentina Address for correspondence: Susanne S. Renner, [email protected] DOI https://doi.org/10.1002/tax.12203 Abstract Coriariaceae comprise only Coriaria, a genus of shrubs with nine species in Australasia (but excluding Australia), five in the Himalayas, Taiwan, the Philippines, and Japan, one in the Mediterranean, and one ranging from Patagonia to Mexico. The sister family, Corynocarpaceae, comprises five species of evergreen trees from New Guinea to New Zealand and Australia. This distribution has long fascinated biogeographers as potential support for Wegener’s theory of continental drift, with alternative scenarios invoking either Antarctic or Beringian range expansions. Here, we present the discovery of pollen grains from Early Campanian (ca. 82 Mya) deposits in Antarctica, which we describe as Coriaripites goodii sp. nov., and newly generated nuclear and plastid molecular data for most of the family’s species and its outgroup. This greatly expands the family’s fossil record and is the so far oldest fossil of the order Cucurbitales. We used the phylogeny, new fossil, and an Oligocene flowering branch assigned to a small subclade of Coriaria to gen- erate a chronogram and to study changes in chromosome number, deciduousness, and andromonoecy. -

I TREE ATTRIBUTE RANKING and PHENOLOGY STUDY: FARMERS
TREE ATTRIBUTE RANKING AND PHENOLOGY STUDY: FARMERS’ KNOWLEDGE OF TREES COMMONLY FOUND ON COFFEE FARMS BORDERING MABIRA FOREST RESERVE IN MUKONO DISTRICT, UGANDA BY MUCHELO RONALD W.OMELI AG332-0855/2009 A DISSERTATION SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR AWARD OF THE DEGREE OF MASTER OF SCIENCE IN RESAERCH METHODS OF JOMO KENYATTA UNIVERSITY OF AGRICULTURE AND TECHNOLOGY September 2011 i Declaration This dissertation is my original work and has not been presented for a degree in any other University Sign: ______________________________ Date: ______________________ Muchelo Ronald Omeli Recommendation This dissertation has been submitted for examination with our approval as the supervisors of the candidate Sign: ______________________________ Date: ______________________ Dr. Samuel Mwalili JKUAT, Kenya Sign: ______________________________ Date: ______________________ Prof. Catherine Muthuri JKUAT, Kenya Sign: ______________________________ Date: ______________________ Prof. Fergus Sinclair ICRAF, Nairobi ii Dedication This dissertation is dedicated to my sisters; Rachel and Sylvia iii Acknowledgment Great appreciation goes to RUFORUM for all the financial support through the scholarship and JKUAT for delivering the course. Special appreciation also goes to Professor Fergus Sinclair and Dr. Anja Gassner for allowing me to be part of their research team and continued technical support. Dr. Mwalili and Prof Muthuri in the same measure are greatly acknowledged. Thanks a lot for sparing your valuable time trying to make sure I am on the right track with this research. Your guidance and constant follow up of my progress has enabled me to make this progress. Appreciation also goes to Genevieve Lamond for and Emilie Smith for their assistance and guidance. Finally I would to extend my appreciation to, NAFORI, ICRAF and CAFNET project team for all the assistance they offered me during my entire time at the institution. -

Molecular Systematics of the Cashew Family (Anacardiaceae) Susan Katherine Pell Louisiana State University and Agricultural and Mechanical College
Louisiana State University LSU Digital Commons LSU Doctoral Dissertations Graduate School 2004 Molecular systematics of the cashew family (Anacardiaceae) Susan Katherine Pell Louisiana State University and Agricultural and Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_dissertations Recommended Citation Pell, Susan Katherine, "Molecular systematics of the cashew family (Anacardiaceae)" (2004). LSU Doctoral Dissertations. 1472. https://digitalcommons.lsu.edu/gradschool_dissertations/1472 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Doctoral Dissertations by an authorized graduate school editor of LSU Digital Commons. For more information, please [email protected]. MOLECULAR SYSTEMATICS OF THE CASHEW FAMILY (ANACARDIACEAE) A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Biological Sciences by Susan Katherine Pell B.S., St. Andrews Presbyterian College, 1995 May 2004 © 2004 Susan Katherine Pell All rights reserved ii Dedicated to my mentors: Marcia Petersen, my mentor in education Dr. Frank Watson, my mentor in botany John D. Mitchell, my mentor in the Anacardiaceae Mary Alice and Ken Carpenter, my mentors in life iii Acknowledgements I would first and foremost like to thank my mentor and dear friend, John D. Mitchell for his unabashed enthusiasm and undying love for the Anacardiaceae. He has truly been my adviser in all Anacardiaceous aspects of this project and continues to provide me with inspiration to further my endeavor to understand the evolution of this beautiful and amazing plant family. -

Antioxidant and Anti-Inflammatory Activities Evaluation of Coriaria Myrtifolia from the North of Morocco
International Food Research Journal 24(2): 498-502 (April 2017) Journal homepage: http://www.ifrj.upm.edu.my Antioxidant and anti-inflammatory activities evaluation of Coriaria myrtifolia from the North of Morocco 1,2Hafsé, M., 2,3Farah, A., 4Mouktadir, J. E. and 1*Fikri-Benbrahim, K. 1Microbial Biotechnology Laboratory. Faculty of Science and Technology. Saiss. Sidi Mohamed Ben Abdellah University. Fez. Morocco 2National Institute of Medicinal and Aromatic Plants. Taounate. Morocco 3Applied Organic Chemistry Laboratory, Faculty of Sciences and Techniques, Sidi Mohamed Ben Abdellah University, P. O. Box 2202 Imouzzer Road, Fez, Morocco. 4Discourse, Society and Creativity: Perception and Implications Laboratory. Faculty of Arts and Humanities Saiss. Sidi Mohamed Ben Abdellah University. Fez. Morocco Article history Abstract Received: 1 January 2016 The present study has examined the chemical composition and evaluated the pharmacological Received in revised form: activities of the ethyl acetate extract of Coriaria myrtifolia. The antioxidant activity was 17 April 2016 Accepted: 21 April 2016 determined by using the diphenyl-picryl-hydrazyl (DPPH) test, and the anti-inflammatory activity was evaluated by using the plantar edema model induced in rabbits by carrageenan. The extract has revealed a significant free-radical scavenging capacity. The plant IC50 value of Keywords 0.016 mg/ml was found less than stated with the Butylated hydroxyl-toluene (BHT) standard (0.025 mg/ml). Administration of the ethyl acetate extract at a dose of 0.013mg/kg wb inhibits Coriaria myrtifolia Chemical composition completely the inflammation. These results have indicated that the C. myrtifolia leaves Antioxidant activity contains bioactive compounds and have a high antioxidant activity as well as interesting anti- Anti-inflammatory activity inflammatory properties, suggesting the usefulness of their extracts to prevent oxidative and inflammatory processes.