Chapter 11: Atmosphere
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Wastewater Technology Fact Sheet: Ammonia Stripping
United States Office of Water EPA 832-F-00-019 Environmental Protection Washington, D.C. September 2000 Agency Wastewater Technology Fact Sheet Ammonia Stripping DESCRIPTION Ammonia stripping is a simple desorption process used to lower the ammonia content of a wastewater stream. Some wastewaters contain large amounts of ammonia and/or nitrogen-containing compounds that may readily form ammonia. It is often easier and less expensive to remove nitrogen from wastewater in the form of ammonia than to convert it to nitrate-nitrogen before removing it (Culp et al., 1978). Ammonia (a weak base) reacts with water (a weak acid) to form ammonium hydroxide. In ammonia stripping, lime or caustic is added to the wastewater until the pH reaches 10.8 to 11.5 standard units which converts ammonium hydroxide ions to ammonia gas according to the following reaction(s): + - NH4 + OH 6 H2O + NH38 Source: Culp, et. al, 1978. Figure 1 illustrates two variations of ammonia FIGURE 1 TWO TYPES OF STRIPPING stripping towers, cross-flow and countercurrent. In TOWERS a cross-flow tower, the solvent gas (air) enters along the entire depth of fill and flows through the packing, as the alkaline wastewater flows it may be more economical to use alternate downward. A countercurrent tower draws air ammonia removal techniques, such as steam through openings at the bottom, as wastewater is stripping or biological methods. Air stripping may pumped to the top of a packed tower. Free also be used to remove many hydrophobic organic ammonia (NH3) is stripped from falling water molecules (Nutrient Control, 1983). droplets into the air stream, then discharged to the atmosphere. -

Ozonedisinfection.Pdf
ETI - Environmental Technology Initiative Project funded by the U.S. Environmental Protection Agency under Assistance Agreement No. CX824652 What is disinfection? Human exposure to wastewater discharged into the environment has increased in the last 15 to 20 years with the rise in population and the greater demand for water resources for recreation and other purposes. Disinfection of wastewater is done to prevent infectious diseases from being spread and to ensure that water is safe for human contact and the environment. There is no perfect disinfectant. However, there are certain characteristics to look for when choosing the most suitable disinfectant: • Ability to penetrate and destroy infectious agents under normal operating conditions; • Lack of characteristics that could be harmful to people and the environment; • Safe and easy handling, shipping, and storage; • Absence of toxic residuals, such as cancer-causing compounds, after disinfection; and • Affordable capital and operation and maintenance (O&M) costs. What is ozone disinfection? One common method of disinfecting wastewater is ozonation (also known as ozone disinfection). Ozone is an unstable gas that can destroy bacteria and viruses. It is formed when oxygen molecules (O2) collide with oxygen atoms to produce ozone (O3). Ozone is generated by an electrical discharge through dry air or pure oxygen and is generated onsite because it decomposes to elemental oxygen in a short amount of time. After generation, ozone is fed into a down-flow contact chamber containing the wastewater to be disinfected. From the bottom of the contact chamber, ozone is diffused into fine bubbles that mix with the downward flowing wastewater. See Figure 1 on page 2 for a schematic of the ozonation process. -
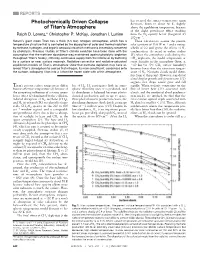
Photochemically Driven Collapse of Titan's Atmosphere
REPORTS has escaped, the surface temperature again Photochemically Driven Collapse decreases, down to about 86 K, slightly of Titan’s Atmosphere above the equilibrium temperature, because of the slight greenhouse effect resulting Ralph D. Lorenz,* Christopher P. McKay, Jonathan I. Lunine from the N2 opacity below (longward of) 200 cm21. Saturn’s giant moon Titan has a thick (1.5 bar) nitrogen atmosphere, which has a These calculations assume the present temperature structure that is controlled by the absorption of solar and thermal radiation solar constant of 15.6 W m22 and a surface by methane, hydrogen, and organic aerosols into which methane is irreversibly converted albedo of 0.2 and ignore the effects of N2 by photolysis. Previous studies of Titan’s climate evolution have been done with the condensation. As noted in earlier studies assumption that the methane abundance was maintained against photolytic depletion (5), when the atmosphere cools during the throughout Titan’s history, either by continuous supply from the interior or by buffering CH4 depletion, the model temperature at by a surface or near surface reservoir. Radiative-convective and radiative-saturated some altitudes in the atmosphere (here, at equilibrium models of Titan’s atmosphere show that methane depletion may have al- ;10 km for 20% CH4 surface humidity) lowed Titan’s atmosphere to cool so that nitrogen, its main constituent, condenses onto becomes lower than the saturation temper- the surface, collapsing Titan into a Triton-like frozen state with a thin atmosphere. -

Introduction to Co2 Chemistry in Sea Water
INTRODUCTION TO CO2 CHEMISTRY IN SEA WATER Andrew G. Dickson Scripps Institution of Oceanography, UC San Diego Mauna Loa Observatory, Hawaii Monthly Average Carbon Dioxide Concentration Data from Scripps CO Program Last updated August 2016 2 ? 410 400 390 380 370 2008; ~385 ppm 360 350 Concentration (ppm) 2 340 CO 330 1974; ~330 ppm 320 310 1960 1965 1970 1975 1980 1985 1990 1995 2000 2005 2010 2015 Year EFFECT OF ADDING CO2 TO SEA WATER 2− − CO2 + CO3 +H2O ! 2HCO3 O C O CO2 1. Dissolves in the ocean increase in decreases increases dissolved CO2 carbonate bicarbonate − HCO3 H O O also hydrogen ion concentration increases C H H 2. Reacts with water O O + H2O to form bicarbonate ion i.e., pH = –lg [H ] decreases H+ and hydrogen ion − HCO3 and saturation state of calcium carbonate decreases H+ 2− O O CO + 2− 3 3. Nearly all of that hydrogen [Ca ][CO ] C C H saturation Ω = 3 O O ion reacts with carbonate O O state K ion to form more bicarbonate sp (a measure of how “easy” it is to form a shell) M u l t i p l e o b s e r v e d indicators of a changing global carbon cycle: (a) atmospheric concentrations of carbon dioxide (CO2) from Mauna Loa (19°32´N, 155°34´W – red) and South Pole (89°59´S, 24°48´W – black) since 1958; (b) partial pressure of dissolved CO2 at the ocean surface (blue curves) and in situ pH (green curves), a measure of the acidity of ocean water. -

National Primary Drinking Water Regulations
National Primary Drinking Water Regulations Potential health effects MCL or TT1 Common sources of contaminant in Public Health Contaminant from long-term3 exposure (mg/L)2 drinking water Goal (mg/L)2 above the MCL Nervous system or blood Added to water during sewage/ Acrylamide TT4 problems; increased risk of cancer wastewater treatment zero Eye, liver, kidney, or spleen Runoff from herbicide used on row Alachlor 0.002 problems; anemia; increased risk crops zero of cancer Erosion of natural deposits of certain 15 picocuries Alpha/photon minerals that are radioactive and per Liter Increased risk of cancer emitters may emit a form of radiation known zero (pCi/L) as alpha radiation Discharge from petroleum refineries; Increase in blood cholesterol; Antimony 0.006 fire retardants; ceramics; electronics; decrease in blood sugar 0.006 solder Skin damage or problems with Erosion of natural deposits; runoff Arsenic 0.010 circulatory systems, and may have from orchards; runoff from glass & 0 increased risk of getting cancer electronics production wastes Asbestos 7 million Increased risk of developing Decay of asbestos cement in water (fibers >10 fibers per Liter benign intestinal polyps mains; erosion of natural deposits 7 MFL micrometers) (MFL) Cardiovascular system or Runoff from herbicide used on row Atrazine 0.003 reproductive problems crops 0.003 Discharge of drilling wastes; discharge Barium 2 Increase in blood pressure from metal refineries; erosion 2 of natural deposits Anemia; decrease in blood Discharge from factories; leaching Benzene -

Amplify Science Earth's Changing Climate
Lawrence Hall of Science has new instructional materials that address the Next Generation Science Standards! Check out these Middle School Units… As just one example, compare Middle School units from three different Hall programs. See for yourself how each program goes about addressing the Middle School NGSS Standards related to Human Impacts and Climate Change, and choose the approach that best meets the needs of your school district. MS NGSS Performance Expectations: Human Impacts and Climate Change • MS-ESS3-2. Analyze and interpret data on natural hazards to forecast future catastrophic events and inform the development of technologies to mitigate their effects. • MS-ESS3-3. Apply scientific principles to design a method for monitoring and minimizing a human impact on the Environment. • MS-ESS3-4. Construct an argument supported by evidence for how increases in human population and per-capita consumption of natural resources impact Earth’s systems. • MS-ESS3-5. Ask questions to clarify evidence of the factors that have caused the rise in global temperatures over the past century. Sample Units from Three Different Hall Programs • Amplify Science—Earth’s Changing Climate: Vanishing Ice Earth’s Changing Climate Engineering Internship • FOSS—Weather and Water • Ocean Sciences Sequence—The Ocean-Atmosphere Connection and Climate Change ©The Regents of the University of California ©The Regents of the University of California Description of two Middle School units from Amplify Science Earth’s Changing Climate: Vanishing Ice and Earth’s Changing Climate Engineering Internship Grade 6-8 Units — requiring at least 19 and 10 45-minute class sessions respectively (two of 27 Middle School Amplify Science units) The Problem: Why is the ice on Earth’s surface melting? Students’ Role: In the role of student climatologists, students investigate what is causing ice on Earth’s surface to melt in order to help the fictional World Climate Institute educate the public about the processes involved. -

Light, Color, and Atmospheric Optics
Light, Color, and Atmospheric Optics GEOL 1350: Introduction To Meteorology 1 2 • During the scattering process, no energy is gained or lost, and therefore, no temperature changes occur. • Scattering depends on the size of objects, in particular on the ratio of object’s diameter vs wavelength: 1. Rayleigh scattering (D/ < 0.03) 2. Mie scattering (0.03 ≤ D/ < 32) 3. Geometric scattering (D/ ≥ 32) 3 4 • Gas scattering: redirection of radiation by a gas molecule without a net transfer of energy of the molecules • Rayleigh scattering: absorption extinction 4 coefficient s depends on 1/ . • Molecules scatter short (blue) wavelengths preferentially over long (red) wavelengths. • The longer pathway of light through the atmosphere the more shorter wavelengths are scattered. 5 • As sunlight enters the atmosphere, the shorter visible wavelengths of violet, blue and green are scattered more by atmospheric gases than are the longer wavelengths of yellow, orange, and especially red. • The scattered waves of violet, blue, and green strike the eye from all directions. • Because our eyes are more sensitive to blue light, these waves, viewed together, produce the sensation of blue coming from all around us. 6 • Rayleigh Scattering • The selective scattering of blue light by air molecules and very small particles can make distant mountains appear blue. The blue ridge mountains in Virginia. 7 • When small particles, such as fine dust and salt, become suspended in the atmosphere, the color of the sky begins to change from blue to milky white. • These particles are large enough to scatter all wavelengths of visible light fairly evenly in all directions. -

Project Horizon Report
Volume I · SUMMARY AND SUPPORTING CONSIDERATIONS UNITED STATES · ARMY CRD/I ( S) Proposal t c• Establish a Lunar Outpost (C) Chief of Ordnance ·cRD 20 Mar 1 95 9 1. (U) Reference letter to Chief of Ordnance from Chief of Research and Devel opment, subject as above. 2. (C) Subsequent t o approval by t he Chief of Staff of reference, repre sentatives of the Army Ballistic ~tissiles Agency indicat e d that supplementar y guidance would· be r equired concerning the scope of the preliminary investigation s pecified in the reference. In particular these r epresentatives requested guidance concerning the source of funds required to conduct the investigation. 3. (S) I envision expeditious development o! the proposal to establish a lunar outpost to be of critical innportance t o the p. S . Army of the future. This eva luation i s appar ently shar ed by the Chief of Staff in view of his expeditious a pproval and enthusiastic endorsement of initiation of the study. Therefore, the detail to be covered by the investigation and the subs equent plan should be as com plete a s is feas ible in the tin1e limits a llowed and within the funds currently a vailable within t he office of t he Chief of Ordnance. I n this time of limited budget , additional monies are unavailable. Current. programs have been scrutinized r igidly and identifiable "fat'' trimmed awa y. Thus high study costs are prohibitive at this time , 4. (C) I leave it to your discretion t o determine the source and the amount of money to be devoted to this purpose. -

51. Astrobiology: the Final Frontier of Science Education
www.astrosociety.org/uitc No. 51 - Summer 2000 © 2000, Astronomical Society of the Pacific, 390 Ashton Avenue, San Francisco, CA 94112. Astrobiology: The Final Frontier of Science Education by Jodi Asbell-Clarke and Jeff Lockwood What (or Whom) Are We Looking For? Where Do We Look? Lessons from Our Past The Search Is On What Does the Public Have to Learn from All This? A High School Curriculum in Astrobiology Astrobiology seems to be all the buzz these days. It was the focus of the ASP science symposium this summer; the University of Washington is offering it as a new Ph.D. program, and TERC (Technical Education Research Center) is developing a high school integrated science course based on it. So what is astrobiology? The NASA Astrobiology Institute defines this new discipline as the study of the origin, evolution, distribution, and destiny of life in the Universe. What this means for scientists is finding the means to blend research fields such as microbiology, geoscience, and astrophysics to collectively answer the largest looming questions of humankind. What it means for educators is an engaging and exciting discipline that is ripe for an integrated approach to science education. Virtually every topic that one deals with in high school science is embedded in astrobiology. What (or Whom) Are We Looking For? Movies and television shows such as Contact and Star Trek have teased viewers with the idea of life on other planets and even in other galaxies. Illustration courtesy of and © 2000 by These fictional accounts almost always deal with intelligent beings that have Kathleen L. -

Planets of the Solar System
Chapter Planets of the 27 Solar System Chapter OutlineOutline 1 ● Formation of the Solar System The Nebular Hypothesis Formation of the Planets Formation of Solid Earth Formation of Earth’s Atmosphere Formation of Earth’s Oceans 2 ● Models of the Solar System Early Models Kepler’s Laws Newton’s Explanation of Kepler’s Laws 3 ● The Inner Planets Mercury Venus Earth Mars 4 ● The Outer Planets Gas Giants Jupiter Saturn Uranus Neptune Objects Beyond Neptune Why It Matters Exoplanets UnderstandingU d t di theth formationf ti and the characteristics of our solar system and its planets can help scientists plan missions to study planets and solar systems around other stars in the universe. 746 Chapter 27 hhq10sena_psscho.inddq10sena_psscho.indd 774646 PDF 88/15/08/15/08 88:43:46:43:46 AAMM Inquiry Lab Planetary Distances 20 min Turn to Appendix E and find the table entitled Question to Get You Started “Solar System Data.” Use the data from the How would the distance of a planet from the sun “semimajor axis” row of planetary distances to affect the time it takes for the planet to complete devise an appropriate scale to model the distances one orbit? between planets. Then find an indoor or outdoor space that will accommodate the farthest distance. Mark some index cards with the name of each planet, use a measuring tape to measure the distances according to your scale, and place each index card at its correct location. 747 hhq10sena_psscho.inddq10sena_psscho.indd 774747 22/26/09/26/09 111:42:301:42:30 AAMM These reading tools will help you learn the material in this chapter. -

Ammonia in Drinking-Water
WHO/SDE/WSH/03.04/01 English only Ammonia in Drinking-water Background document for development of WHO Guidelines for Drinking-water Quality _______________________ Originally published in Guidelines for drinking-water quality, 2nd ed. Vol. 2. Health criteria and other supporting information. World Health Organization, Geneva, 1996. © World Health Organization 2003 All rights reserved. Publications of the World Health Organization can be obtained from Marketing and Dissemination, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 2476; fax: +41 22 791 4857; email: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to Publications, at the above address (fax: +41 22 791 4806; email: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. The World Health Organization does not warrant that the information contained in this publication is complete and correct and shall not be liable for any damages incurred as a result of its use. -

Earth Science SCIH 041 055 Credits: 0.5 Units / 5 Hours / NCAA
UNIVERSITY OF NEBRASKA HIGH SCHOOL Earth Science SCIH 041 055 Credits: 0.5 units / 5 hours / NCAA Course Description Have you ever wondered where marble comes from? or how deep the ocean is? or why it rains more in areas near the Equator than in other places? In this course students will study a variety of topics designed to give them a better understanding of the planet on which we live. They will study the composition of Earth including minerals and different rock types, weathering and erosion processes, mass movements, and surface and groundwater. They will also explore Earth's atmosphere and oceans, including storms, climate and ocean movements, plate tectonics, volcanism, earthquakes, mountain building, and geologic time. This course concludes with an in-depth look at the connections between our Earth's vast resources and the human population's dependence and impact on them. Graded Assessments: 5 Unit Evaluations; 2 Projects; 2 Proctored Progress Tests, 5 Teacher Connect Activities Course Objectives When you have completed the materials in this course, you should be able to: 1. Describe how Earth materials move through geochemical cycles (carbon, nitrogen, oxygen) resulting in chemical and physical changes in matter. 2. Understand the relationships among Earth’s structure, systems, and processes. 3. Describe how heat convection in the mantle propels the plates comprising Earth’s surface across the face of the globe (plate tectonics). 4. Evaluate the impact of human activity and natural causes on Earth’s resources (groundwater, rivers, land, fossil fuels). 5. Describe the relationships among the sources of energy and their effects on Earth’s systems.