Uva-DARE (Digital Academic Repository)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Singapore Avifauna Vol 23 No 9
SSIINNGGAAPPOORREE AAVVIIFFAAUUNNAA A monthly bulletin of the Nature Society (Singapore) Bird Group Volume 23 Published by Nature Society (Singapore) Bird Group, 510 Geylang Road, #02-05, The Sunflower, Singapore 389466. Number 9 Tel : 67412036, Fax : 67410871, Email : [email protected] , Website : http://www.nss.org.sg MICA(P) 239/11/2005 CONTENTS NSS Bird Group 1 Bird Report: September 2009 Compiled by Lim Kim Seng Chairman 15 Autumn Raptor Migration - Early arrivals for September 2009 Alan OwYong Compiled by Alan OwYong, edited by Kenneth Kee ([email protected] ) 16 Report on the 6 th Fall Migration Bird Census By Lim Kim Seng Vice-Chairman Ho Hua Chew ([email protected] ) SINAV Secretary Editorial Committee Willie Foo ([email protected] ) Lim Kim Chuah, Lim Kim Seng, Yong Ding Li, Andrew Chow, Albert Low Rail Babbler Eupetes macrocerus at Panti Forest Reserve on 20 September 2009 By Chong Boon Leong Nature Society (Singapore) is the national partner of Singapore Avifauna Volume 23 No 9 _____________________________________________________________________________ Bird Report September 2009 By Lim Kim Seng SINGAPORE HIGHLIGHTS Lesser Sand Plovers Charadrius mongolus at Changi Cove on 21 Sep 09 By Lee Tiah Kee The winter was well and truly on its way as evidenced by the presence of an additional 27 new migrants, for a grand total of 43 species in the winter to date. Among the more spectacular species were a stunned (but well) Black-backed Kingfisher at Toa Payoh Central on 24th, a new early date by 4 days, and our 12th record of Brown-streaked Flycatcher , a juvenile seen and photographed at Chinese Garden on 1st and 2nd, and our second record in two months. -

A Complete Species-Level Molecular Phylogeny For
Author's personal copy Available online at www.sciencedirect.com Molecular Phylogenetics and Evolution 47 (2008) 251–260 www.elsevier.com/locate/ympev A complete species-level molecular phylogeny for the ‘‘Eurasian” starlings (Sturnidae: Sturnus, Acridotheres, and allies): Recent diversification in a highly social and dispersive avian group Irby J. Lovette a,*, Brynn V. McCleery a, Amanda L. Talaba a, Dustin R. Rubenstein a,b,c a Fuller Evolutionary Biology Program, Laboratory of Ornithology, Cornell University, Ithaca, NY 14950, USA b Department of Neurobiology and Behavior, Cornell University, Ithaca, NY 14850, USA c Department of Integrative Biology and Museum of Vertebrate Zoology, University of California, Berkeley, CA 94720, USA Received 2 August 2007; revised 17 January 2008; accepted 22 January 2008 Available online 31 January 2008 Abstract We generated the first complete phylogeny of extant taxa in a well-defined clade of 26 starling species that is collectively distributed across Eurasia, and which has one species endemic to sub-Saharan Africa. Two species in this group—the European starling Sturnus vulgaris and the common Myna Acridotheres tristis—now occur on continents and islands around the world following human-mediated introductions, and the entire clade is generally notable for being highly social and dispersive, as most of its species breed colonially or move in large flocks as they track ephemeral insect or plant resources, and for associating with humans in urban or agricultural land- scapes. Our reconstructions were based on substantial mtDNA (4 kb) and nuclear intron (4 loci, 3 kb total) sequences from 16 species, augmented by mtDNA NDII gene sequences (1 kb) for the remaining 10 taxa for which DNAs were available only from museum skin samples. -

Using Geolocator Tracking Data and Ringing Archives to Validate Citizen-Science Based Seasonal Predictions of Bird Distribution in a Data-Poor Region
Global Ecology and Conservation 24 (2020) e01215 Contents lists available at ScienceDirect Global Ecology and Conservation journal homepage: http://www.elsevier.com/locate/gecco Original Research Article Using geolocator tracking data and ringing archives to validate citizen-science based seasonal predictions of bird distribution in a data-poor region * Wieland Heim a, , Ramona J. Heim a, Ilka Beermann a, Oleg A. Burkovskiy b, Yury Gerasimov c, Pavel Ktitorov d, e, Kiyoaki Ozaki f, Ilya Panov g, h, Martha Maria Sander i, Sissel Sjoberg€ j, Sergei M. Smirenski k, Alexander Thomas l, Anders P. Tøttrup o, Ivan M. Tiunov m, Mikkel Willemoes n, Norbert Holzel€ a, Kasper Thorup j, Johannes Kamp a, p a Institute of Landscape Ecology, University of Münster, Germany b Sakhalin Energy, Investment Company LTD, Yuzhno-Sakhalinsk, Russia c Kamchatka Department of Pacific Geographical Institute, Far-eastern Branch of Russian Academy of Science, Petropavlovsk- Kamchatskiy, Russia d Institute of Biological Problems of the North, Far-eastern Branch of Russian Academy of Science, Magadan, Russia e Birds Russia, Yuzhno-Sakhalinsk, Russia f Yamashina Institute for Ornithology, Chiba, Japan g Bird Ringing Centre of Russia, IPEE RAS, Russia h Biological Station Rybachy, ZIN RAS, Russia i Department of Life Sciences and Systems Biology, University of Turin, Italy j Center for Macroecology, Evolution and Climate, Globe Institute, University of Copenhagen, Denmark k Muraviovka Park for Sustainable Land Use, Blagoveshchensk, Russia l School of Environmental Science and Engineering, Southern University of Science and Technology, Shenzhen, China m Federal Scientific Center of the East Asia Terrestrial Biodiversity, Far Eastern Branch, Russian Academy of Sciences, Vladivostok, Russia n Department of Biology, Lund University, Sweden o Natural History Museum of Denmark, University of Copenhagen, Denmark p University of Gottingen,€ Department of Conservation Biology, Bürgerstr. -

Thailand Highlights 14Th to 26Th November 2019 (13 Days)
Thailand Highlights 14th to 26th November 2019 (13 days) Trip Report Siamese Fireback by Forrest Rowland Trip report compiled by Tour Leader: Forrest Rowland Trip Report – RBL Thailand - Highlights 2019 2 Tour Summary Thailand has been known as a top tourist destination for quite some time. Foreigners and Ex-pats flock there for the beautiful scenery, great infrastructure, and delicious cuisine among other cultural aspects. For birders, it has recently caught up to big names like Borneo and Malaysia, in terms of respect for the avian delights it holds for visitors. Our twelve-day Highlights Tour to Thailand set out to sample a bit of the best of every major habitat type in the country, with a slight focus on the lush montane forests that hold most of the country’s specialty bird species. The tour began in Bangkok, a bustling metropolis of winding narrow roads, flyovers, towering apartment buildings, and seemingly endless people. Despite the density and throng of humanity, many of the participants on the tour were able to enjoy a Crested Goshawk flight by Forrest Rowland lovely day’s visit to the Grand Palace and historic center of Bangkok, including a fun boat ride passing by several temples. A few early arrivals also had time to bird some of the urban park settings, even picking up a species or two we did not see on the Main Tour. For most, the tour began in earnest on November 15th, with our day tour of the salt pans, mudflats, wetlands, and mangroves of the famed Pak Thale Shore bird Project, and Laem Phak Bia mangroves. -

NSS Bird Group Report – November 2019
NSS Bird Group Report – November 2019 By Geoff Lim, Alan Owyong (compiler), Tan Gim Cheong (ed.). November was spectacular, with the first record of two species – the Fairy Pitta and Shikra at the Central Catchment Nature Reserve; an Oriental Dwarf Kingfisher (the locally extinct rufous- backed subspecies), found inside a camera shop in the city; and, a rare Red-footed Booby at St John’s Island. Also, it was and has always been a great month to spot migrating raptors in southern Singapore. A Fairy’s Visitation in November The first Fairy Pitta discovered in Singapore on 8 Nov 2019 – photo by Francis Yap. On 8 November 2019, Francis Yap and Richard White were en route to Jelutong Tower, when the duo spotted a paler than usual pitta along the trail under the darkening morning sky as a storm threatened from Sumatra. When Francis managed to regain phone reception and were able to refer to other photos on the internet, the two confirmed that they had Singapore’s first record of the Fairy Pitta, Pitta nympha. Francis’ electrifying account can be accessed here. The Fairy Pitta stopped over for a week, with daily records from 8-13 November 2019. 1 The Fairy Pitta has been recognised as part of a superspecies comprising the Blue-winged Pitta, P. moluccensis, Mangrove Pitta, P. megarhyncha, and Indian Pitta, P. brachyura (Lambert & Woodcock, 1996:162), hence the superficial resemblance with one another. BirdLife has classified the species as Vulnerable, with key threats being habitat loss and conversion, as well as local trapping pressure (BirdLife, 2019). -
ORL 5.1 Hypothetical Spp Final Draft01a.Xlsx
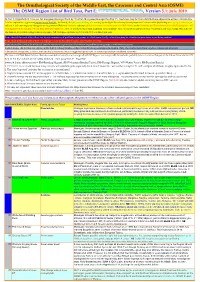
The Ornithological Society of the Middle East, the Caucasus and Central Asia (OSME) The OSME Region List of Bird Taxa, Part E: , Version 5.1: July 2019 In Part E, Hypothetical Taxa, we list non-passerines (prefixed by 'N') first, then passerines (prefixed by 'P'). Such taxa may be from distributions adjacent to or have extended to A fuller explanation is given in Explanation of the ORL, but briefly, Bright green shading of a row (eg Syrian Ostrich) indicates former presence of a taxon in the OSME Region. Light gold shading in column A indicates sequence change from the previous ORL issue. Red font indicates added information since the previous ORL version or the Conservation Threat Status (Critically Endangered = CE, Endangered = E, Vulnerable = V and Data Deficient = DD only). Not all synonyms have been examined. Serial numbers (SN) are merely an administrative convenience and may change. Please do not cite them in any formal correspondence or papers. NB: Compass cardinals (eg N = north, SE = southeast) are used. Rows shaded thus and with yellow text denote summaries of problem taxon groups in which some closely-related taxa may be of indeterminate status or are being studied. Rows shaded thus and with yellow text indicate recent or data-driven major conservation concerns. Rows shaded thus and with white text contain additional explanatory information on problem taxon groups as and when necessary. English names shaded thus are species on BirdLife Tracking Database, http://seabirdtracking.org/mapper/index.php. Only a few individuals from very few colonies are involved. A broad dark orange line, as below, indicates the last taxon in a new or suggested species split, or where sspp are best considered separately. -

K Here for the Full Trip Report
Capped Langur , Small Pratincole , WhiteWhite----wingedwinged Ducks , Gaur , Sultan Tit , Great Hornbill andand Pied Falconet ; Nameri Here a couple of ducks had now turned up , and while we were watching them , a Gaur suddenly came out of the forest for a drink and some fresh grass from the meadow surrounding the lake. We also saw several Wild Boars and a small group of Northern Red Muntjacs here , not to mention a Great Hornbill , Sultan Tits and a small party of Scarlet Minivets. No doubt this was a fantastic place , and there is no telling what could have been seen if more time had been spent here. Back in the camp we enjoyed yet another good meal , before driving a bit up river where this afternoons boat ride was to begin. We didn’t really see all that many birds while rafting on the river , but even so it was a nice experience to watch the beautiful landscape pass by in a leisurely pace – not exactly white water rafting this! Of course , there were a few avian highlights as well , including lots of Small Pratincoles , a couple of Crested Kingfisher and some nice River Lapwings , but we somehow managed to dip out on Ibisbill. We walked back to the camp as the sun was setting , but didn’t add anything new to our list , though a couple of Brown Hawk Owls put on quite a show for Erling , who was the first one to get back. We tried again with some spotlighting in the evening , and heard a calling Oriental Scops Owl not to far from the road but still impossible to see. -

Mai Po Nature Reserve Management Plan: 2019-2024
Mai Po Nature Reserve Management Plan: 2019-2024 ©Anthony Sun June 2021 (Mid-term version) Prepared by WWF-Hong Kong Mai Po Nature Reserve Management Plan: 2019-2024 Page | 1 Table of Contents EXECUTIVE SUMMARY ................................................................................................................................................... 2 1. INTRODUCTION ..................................................................................................................................................... 7 1.1 Regional and Global Context ........................................................................................................................ 8 1.2 Local Biodiversity and Wise Use ................................................................................................................... 9 1.3 Geology and Geological History ................................................................................................................. 10 1.4 Hydrology ................................................................................................................................................... 10 1.5 Climate ....................................................................................................................................................... 10 1.6 Climate Change Impacts ............................................................................................................................. 11 1.7 Biodiversity ................................................................................................................................................ -

Quantitative Investigations on Bird Communities in Different Habitats in the Orkhon-Selenge-Valley in Northern Mongolia
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Erforschung biologischer Ressourcen der Mongolei Institut für Biologie der Martin-Luther-Universität / Exploration into the Biological Resources of Halle-Wittenberg Mongolia, ISSN 0440-1298 2005 Quantitative Investigations on Bird Communities in Different Habitats in the Orkhon-Selenge-Valley in Northern Mongolia Tobias Stenzel Martin-Luther-Universität, [email protected] Michael Stubbe Martin-Luther-Universität, [email protected] R. Samjaa National University of Mongolia, [email protected] S. Gombobaatar National University of Mongolia Follow this and additional works at: http://digitalcommons.unl.edu/biolmongol Part of the Asian Studies Commons, Biodiversity Commons, Desert Ecology Commons, Environmental Sciences Commons, Nature and Society Relations Commons, Ornithology Commons, Other Animal Sciences Commons, Other Ecology and Evolutionary Biology Commons, Population Biology Commons, and the Zoology Commons Stenzel, Tobias; Stubbe, Michael; Samjaa, R.; and Gombobaatar, S., "Quantitative Investigations on Bird Communities in Different Habitats in the Orkhon-Selenge-Valley in Northern Mongolia" (2005). Erforschung biologischer Ressourcen der Mongolei / Exploration into the Biological Resources of Mongolia, ISSN 0440-1298. 127. http://digitalcommons.unl.edu/biolmongol/127 This Article is brought to you for free and open access by the Institut für Biologie der Martin-Luther-Universität Halle-Wittenberg at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Erforschung biologischer Ressourcen der Mongolei / Exploration into the Biological Resources of Mongolia, ISSN 0440-1298 by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. In: Proceedings of the symposium ”Ecosystem Research in the Arid Environments of Central Asia: Results, Challenges, and Perspectives,” Ulaanbaatar, Mongolia, June 23-24, 2004. -

2012 Vietnam Tour Species List
Eagle-Eye Tours www.eagle-eye.com [email protected] 1-800-373-5678 VIETNAM 2012 March BIRD SPECIES No. Common Name Latin Name Seen or Heard PHEASANTS AND PARTRIDGES 1 Rufous-throated Partridge Arborophila rufogularis h 2 Scaly-breasted Partridge Arborophila chloropus s 3 Chestnut-necklaced Partridge Arborophila charltonii h 4 Red Junglefowl Gallus gallus s 5 Silver Pheasant Lophura nycthemera s 6 Siamese Fireback Lophura diardi s 7 Germain's Peacock-Pheasant Polyplectron germaini s 8 Green Peafowl Pavo muticus s GREBES 9 Little Grebe Tachybaptus ruficollis s CORMORANTS AND SHAGS 10 Little Cormorant Phalacrocorax niger s ANHINGAS 11 Oriental Darter Anhinga melanogaster s HERONS, EGRETS, AND BITTERNS 12 Yellow Bittern Ixobrychus sinensis s 13 Black Bittern Dupetor flavicollis s 14 Gray Heron Ardea cinerea s 15 Purple Heron Ardea purpurea s 16 Eastern Great Egret Ardea modesta s 17 Intermediate Egret Egretta intermedia s 18 Little Egret Egretta garzetta s 19 (Eastern) Cattle Egret Bubulcus ibis (coromandus) s 20 Chinese Pond-Heron Ardeola bacchus s 21 Javan Pond-Heron Ardeola speciosa s 22 Striated Heron Butorides striata s 23 Black-crowned Night-Heron Nycticorax nycticorax s STORKS 24 Woolly-necked Stork Ciconia episcopus s 25 Lesser Adjutant Leptoptilos javanicus s OSPREY 26 Osprey Pandion haliaetus s HAWKS, EAGLES, AND KITES 27 Black Baza Aviceda leuphotes s 28 Oriental (Crested) Honey-buzzard Pernis ptilorhynchus s 29 Black-shouldered Kite Elanus caeruleus s Page 1 of 9 No. Common Name Latin Name Seen or Heard 30 Black-eared Kite -

Thailand Custom Tour 29 January -13 February, 2017
Tropical Birding Trip Report THAILAND JANUARY-FEBRUARY, 2017 Thailand custom tour 29 January -13 February, 2017 TOUR LEADER: Charley Hesse Report by Charley Hesse. Photos by Charley Hesse & Laurie Ross. All photos were taken on this tour When it comes to vacation destinations, Thailand has it all: great lodgings, delicious food, scenery, good roads, safety, value for money and friendly people. In addition to both its quantity & quality of birds, it is also one of the most rapidly evolving destinations for bird photography. There are of course perennial favourite locations that always produce quality birds, but year on year, Thailand comes up with more and more fantastic sites for bird photography. On this custom tour, we followed the tried and tested set departure itinerary and found an impressive 420 species of birds and 16 species of mammals. Some of the highlights included: Spoon-billed Sandpiper and Nordmann’s Greenshank around Pak Thale; Wreathed Hornbill, Long-tailed & Banded Broadbills inside Kaeng Krachan National Park; Rosy, Daurian & Spot-winged Starlings at a roost site just outside; Kalij Pheasant, Scaly-breasted & Bar-backed Partridges at a private photography blind nearby; Siamese Fireback and Great Hornbill plus Asian Elephant & Malayan Porcupine at Khao Yai National Park; countless water birds at Bueng Boraphet; a myriad of montane birds at Doi Inthanon; Giant Nuthatch at Doi Chiang Dao; Scarlet-faced Liocichla at Doi Ang Khang; Hume’s Pheasant & Spot-breasted Parrotbill at Doi Lang; Yellow-breasted Buntings at Baan Thaton; and Baikal Bush-Warbler & Ferruginous Duck at Chiang Saen. It was a truly unforgettable trip. www.tropicalbirding.com +1-409-515-9110 [email protected] Tropical Birding Trip Report THAILAND JANUARY-FEBRUARY, 2017 29th January – Bangkok to Laem Pak Bia After a morning arrival in Bangkok, we left the sprawling metropolis on the overhead highways, and soon had our first birding stop at the Khok Kham area of Samut Sakhon, the neighbouring city to Bangkok. -

Taxonomic Updates to the Checklists of Birds of India, and the South Asian Region—2020
12 IndianR BI DS VOL. 16 NO. 1 (PUBL. 13 JULY 2020) Taxonomic updates to the checklists of birds of India, and the South Asian region—2020 Praveen J, Rajah Jayapal & Aasheesh Pittie Praveen, J., Jayapal, R., & Pittie, A., 2020. Taxonomic updates to the checklists of birds of India, and the South Asian region—2020. Indian BIRDS 16 (1): 12–19. Praveen J., B303, Shriram Spurthi, ITPL Main Road, Brookefields, Bengaluru 560037, Karnataka, India. E-mail: [email protected]. [Corresponding author.] Rajah Jayapal, Sálim Ali Centre for Ornithology and Natural History, Anaikatty (Post), Coimbatore 641108, Tamil Nadu, India. E-mail: [email protected] Aasheesh Pittie, 2nd Floor, BBR Forum, Road No. 2, Banjara Hills, Hyderabad 500034, Telangana, India. E-mail: [email protected] Manuscript received on 05 January 2020 April 2020. Introduction taxonomic policy of our India Checklist, in 2020 and beyond. The first definitive checklist of the birds of India (Praveen et .al In September 2019 we circulated a concept note, on alternative 2016), now in its twelfth version (Praveen et al. 2020a), and taxonomic approaches, along with our internal assessment later that of the Indian Subcontinent, now in its eighth version of costs and benefits of each proposition, to stakeholders of (Praveen et al. 2020b), and South Asia (Praveen et al. 2020c), major global taxonomies, inviting feedback. There was a general were all drawn from a master database built upon a putative list of support to our first proposal, to restrict the consensus criteria to birds of the South Asian region (Praveen et al. 2019a). All these only eBird/Clements and IOC, and also to expand the scope to checklists, and their online updates, periodically incorporating all the taxonomic categories, from orders down to species limits.