East Pond Study
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Following Document Comes to You From
MAINE STATE LEGISLATURE The following document is provided by the LAW AND LEGISLATIVE DIGITAL LIBRARY at the Maine State Law and Legislative Reference Library http://legislature.maine.gov/lawlib Reproduced from scanned originals with text recognition applied (searchable text may contain some errors and/or omissions) ACTS AND RESOLVES AS PASSED BY THE Ninetieth and Ninety-first Legislatures OF THE STATE OF MAINE From April 26, 1941 to April 9, 1943 AND MISCELLANEOUS STATE PAPERS Published by the Revisor of Statutes in accordance with the Resolves of the Legislature approved June 28, 1820, March 18, 1840, March 16, 1842, and Acts approved August 6, 1930 and April 2, 193I. KENNEBEC JOURNAL AUGUSTA, MAINE 1943 PUBLIC LAWS OF THE STATE OF MAINE As Passed by the Ninety-first Legislature 1943 290 TO SIMPLIFY THE INLAND FISHING LAWS CHAP. 256 -Hte ~ ~ -Hte eOt:l:llty ffi' ft*; 4tet s.e]3t:l:ty tfl.a.t mry' ~ !;;llOWR ~ ~ ~ ~ "" hunting: ffi' ftshiRg: Hit;, ffi' "" Hit; ~ mry' ~ ~ ~, ~ ft*; eounty ~ ft8.t rett:l:rRes. ~ "" rC8:S0R8:B~e tffi:re ~ ft*; s.e]38:FtaFe, ~ ~ ffi" 5i:i'ffi 4tet s.e]3uty, ~ 5i:i'ffi ~ a-5 ~ 4eeme ReCCSS8:F)-, ~ ~ ~ ~ ~ ffi'i'El, 4aH ~ eRtitles. 4E; Fe8:50nable fee5 ffi'i'El, C!E]3C::lSCS ~ ft*; sen-ices ffi'i'El, ~ ft*; ffi4s, ~ ~ ~ ~ -Hte tFeasurcr ~ ~ eouRty. BefoFc tfte sffi4 ~ €of' ~ ~ 4ep i:tt;- ~ ffle.t:J:.p 8:s.aitional e1E]3cfisc itt -Hte eM, ~ -Hte ~ ~~' ~, ftc ~ ~ -Hte conseRt ~"" lIiajority ~ -Hte COt:l:fity COfi111'lissioReFs ~ -Hte 5a+4 coufity. Whenever it shall come to the attention of the commis sioner -

Belgrade Lakes Water Quality
“Keeping Great Pond $$Golden$$: The Belgrade Lakes Project” D. Whitney King, Cathy Bevier, Denise Bruesewitz, Russ Cole, Sahan Dissanayake, Philip Nyhus, James Fleming, Michael Donihue, Bruce Rueger1, Maggie Shannon2, Charlie Baeder, Peter Kallin3, Kathi Wall, Lisa Hallee4, Brenda Fekete 1Colby College, 2Maine Lakes Society, 3Belgrade Regional Conservation Alliance, 4Maine Lakes Resource Center Supported by National Science Foundation award #EPS-0904155 to Maine EPSCoR at the University of Maine http://web.colby.edu/epscor/ VLMP Links of Interest Interactive Lake Data Page: http://www.mapsforgood.org/mlrc/ Water flow animation: https://rossdonihue.cartodb.com/viz/a16b9a20-f516-11e4-8280-0e018d66dc29/embed_map Live Buoy Data: http://web.colby.edu/lakes/ A Maine Sense of Place - with economic value of billions of dollars Stakeholder Perceptions of Water Quality Hydrology of the Belgrade Lake Chain $$$ 48 $$$ 12 17 28 22 East Pond North Pond McGrath Pond $$$ Salmon Lake Great $$$ Pond 4 8 3 $$$ Long Pond - North Long Pond - South Snow Pond Life is controlled by the limiting reagent How many bicycles can I make from three wheels and two frames????? Volunteer Science 1988 Anoxic Factor (AF) = (anoxic area/ lake area) * days of anoxia AF provides an estimate of internal phosphorus loading 12 mg P/m2 day of anoxic area <10 is good, 20 is a concern, 30 is bad, 50+ is awful Oxygen above 2 ppm Depth of anoxia Oxygen below 2 ppm Nurnberg, G. K. 2004 ScienceWorld 4:42 Clear Water Lake China Lake Long Pond station 1 Great Pond Station 2 Deep Lakes good concern bad awful Shallow Lakes Belgrade Lakes Nurnberg, G. -

Real Estate Tax Commitment Book - 18.900 09/09/2016 9:46 AM 2016 Commitment Page 1 Account Name & Address Land Building Exemption Assessment Tax 2000 0 0 0 0 0.00
ANSON Real Estate Tax Commitment Book - 18.900 09/09/2016 9:46 AM 2016 Commitment Page 1 Account Name & Address Land Building Exemption Assessment Tax 2000 0 0 0 0 0.00 ANSON ME 04911 1992 0 0 0 0 0.00 ANSON ME 04911 1993 0 0 0 0 0.00 ANSON ME 04911 2019 0 0 0 0 0.00 ANSON ME 04911 2018 0 0 0 0 0.00 ANSON ME 04911 1994 0 0 0 0 0.00 ANSON ME 04911 1995 0 0 0 0 0.00 ANSON ME 04911 1997 0 0 0 0 0.00 ANSON ME 04911 Land Building Exempt Total Tax Page Totals: 0 0 0 0 0.00 Subtotals: 0 0 0 0 0.00 ANSON Real Estate Tax Commitment Book - 18.900 09/09/2016 9:46 AM 2016 Commitment Page 2 Account Name & Address Land Building Exemption Assessment Tax 1998 0 0 0 0 0.00 ANSON ME 04911 1999 0 0 0 0 0.00 ANSON ME 04911 1002 200 ELM REALTY, LLC 10,340 0 0 10,340 195.43 200 BERKELEY ST, 17TH Acres 0.33 FL BOSTON MA 02116 Soft: 0.00 0 Mixed: 0.00 0 Hard: 0.00 0 001 094 B4894P354 03/24/2015 1003 200 ELM REALTY, LLC 17,490 89,740 0 107,230 2,026.65 200 BERKELEY ST, 17TH Acres 0.34 FL BOSTON MA 02116 Soft: 0.00 0 Mixed: 0.00 0 Hard: 0.00 0 63 MAIN ST 001 095 B4894P354 03/24/2015 2001 ABBOTT, KATIE 27,740 20,660 0 48,400 914.76 PO BOX 496 Acres 6.24 ANSON ME 04911-0496 Soft: 0.00 0 Mixed: 0.00 0 Hard: 0.00 0 212 RIVER ROAD 015 063 1A B4833P293 08/05/2014 955 ABBOTT, KATIE 9,380 37,200 15,000 31,580 596.86 PO BOX 496 Acres 0.22 01 Homestead Exempt ANSON ME 04911-0496 Soft: 0.00 0 Mixed: 0.00 0 Hard: 0.00 0 36 RANDALL ST 003 055 B4748P146 12/12/2013 1238 ABBOTT, LINDA 0 14,700 14,700 0 0.00 17 HILLTOP RD 01 Homestead Exempt ANSON ME 04911-0000 17 HILLTOP RD 003 017 ON Land Building Exempt Total Tax Page Totals: 64,950 162,300 29,700 197,550 3,733.70 Subtotals: 64,950 162,300 29,700 197,550 3,733.70 ANSON Real Estate Tax Commitment Book - 18.900 09/09/2016 9:46 AM 2016 Commitment Page 3 Account Name & Address Land Building Exemption Assessment Tax 102 ABDELRIHIM, MOHAMED A. -

Real Estate Tax Commitment Book
Embden Real Estate Tax Commitment Book - 12.460 06/12/2013 12:03 PM 2013 Tax Commitment Page 1 Account Name & Address Land Building Exemption Assessment Tax 807 880 EAST SHORE REALTY 146,500 228,200 0 374,700 4,668.76 TRUST BAUMANN, EDWARD Acres 1.07 (TRUSTEE) 5 MILK STREET Soft: 0.00 0 2,334.38 (1) SUITE 3 Mixed: 0.00 0 2,334.38 (2) PORTLAND, ME Hard: 0.00 0 04101-4170 880 EAST SHORE ROAD 016-018 B4396P226 05/20/2011 B3302P354 05/06/2004 Land Building Exempt Total Tax Page Totals: 146,500 228,200 0 374,700 4,668.76 Subtotals: 146,500 228,200 0 374,700 4,668.76 Embden Real Estate Tax Commitment Book - 12.460 06/12/2013 12:03 PM 2013 Tax Commitment Page 2 Account Name & Address Land Building Exemption Assessment Tax 2 ABBOTT, MARK 15,600 3,700 0 19,300 240.48 PO BOX 56 Acres 1.47 READFIELD ME 04355 Soft: 0.00 0 120.24 (1) Mixed: 0.00 0 120.24 (2) Hard: 0.00 0 429 KENNEBEC RIVER ROAD 006-040 B2784P299 04/19/2001 B1235P73 01/02/1986 905 ABDELRIHIM, MOHAMED A. 12,000 28,100 0 40,100 499.65 & RHONDA L. 38 ASH STREET Acres 1.00 MADISON, ME 04950 Soft: 0.00 0 249.83 (1) Mixed: 0.00 0 249.82 (2) Hard: 0.00 0 558 KENNEBEC RIVER ROAD 006-054-001 B4234P180 09/16/2009 B1825P92 09/25/1992 4 ADAMS, CLAY A. -
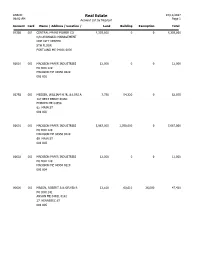
Real Estate 10/12/2017 09:02 AM Account List by Map/Lot Page 1 Account Card Name / Address / Location / Land Building Exemption Total
ANSON Real Estate 10/12/2017 09:02 AM Account List by Map/Lot Page 1 Account Card Name / Address / Location / Land Building Exemption Total 01338 001 CENTRAL MAINE POWER CO 4,339,800 0 0 4,339,800 C/O AVANGRID MANAGEMENT ONE CITY CENTER 5TH FLOOR PORTLAND ME 04101 0000 01610 001 MADISON PAPER INDUSTRIES 12,000 0 0 12,000 PO BOX 129 MADISON ME 04950 0129 001 001 01758 001 MESSER, WILLIAM H JR. & LORI A 7,750 54,320 0 62,070 187 BERT BERRY ROAD EMBDEN ME 04958 62 MAIN ST 001 002 01601 001 MADISON PAPER INDUSTRIES 5,967,000 1,700,000 0 7,667,000 PO BOX 129 MADISON ME 04950 0129 60 MAIN ST 001 003 01602 001 MADISON PAPER INDUSTRIES 12,000 0 0 12,000 PO BOX 129 MADISON ME 04950 0129 001 004 00600 001 MASON, ROBERT A & SYLVIA R 12,810 60,610 26,000 47,420 PO BOX 141 ANSON ME 04911 0141 27 KENNEBEC ST 001 005 ANSON Real Estate 10/12/2017 09:02 AM Account List by Map/Lot Page 2 Account Card Name / Address / Location / Land Building Exemption Total 00268 001 DAGGETT, GERALD 10,950 66,490 20,000 57,440 PO BOX 275 ANSON ME 04911 0275 25 KENNEBEC ST 001 006 00279 001 JACYSSIN, KYLE E. 12,650 46,380 0 59,030 39 LINDA RD TIVERTON RI 02878 0000 21 KENNEBEC ST 001 007 01204 001 WILLETTE, PRISCILLA A 10,200 62,620 26,000 46,820 PO BOX 266 ANSON ME 04911 0266 17 KENNEBEC ST 001 008 01597 001 ANSON TOWN OF 20,710 145,240 165,950 0 PO BOX 297 ANSON ME 04911 0297 70 MAIN ST 001 009 01658 001 PARSON'S REALTY CO INC 15,590 92,820 0 108,410 PO BOX 460 YORK ME 03909 0460 72 MAIN ST 001 009 B 00715 001 MORAN, STEPHEN M 8,720 40,660 0 49,380 17 HILLTOP RD MADISON ME 04950 0000 16 ARNOLDS LN 001 010 ANSON Real Estate 10/12/2017 09:02 AM Account List by Map/Lot Page 3 Account Card Name / Address / Location / Land Building Exemption Total 00524 001 LEEMAN, DIANE B 11,490 49,030 20,000 40,520 PO BOX 226 ANSON ME 04911 0226 20 ARNOLDS LN 001 011 00781 001 LEEMAN, DIANE B. -

Waterville Caniba Naturals
in Maine June 6, 2018 Special Advertising Supplement Kennebec Journal Morning Sentinel 2 Wednesday, June 6, 2018 _______________________________________________________Advertising Supplement • Kennebec Journal • Morning Sentinel INDEX OF ADVERTISERS AUTOMOTIVE CANNABIS CONNECTION St. Joseph Maronite Catholic Columbia Classic Cars...................13 Cannabis Connection Directories 70-71 Church ..........................................65 Skowhegan & Waterville Caniba Naturals ..............................70 St. Mary............................................65 Tire Center ....................................26 Cannabis Healing Center, The .......70 St. Michael Parish ...........................65 Father Jimmy’s ...............................70 Sugarloaf Christian Ministry ..........65 ANIMALS & Harry Brown’s Farm .......................70 Summer Worship Directory ...........65 PETS Homegrown Healthcare Union Church of Belgrade Lakes Paws and Claws........................19, 57 Apothecary & Learning Center ...71 United Methodist Church ............65 Companion Animal Clinic ..............57 Integr8 Health..................................70 Unity United Methodist Church .....65 Hometown Veterinary Care ............57 Limited Edition Farm, LLC - Vassalboro United Methodist Kennebec Veterinary Care .............57 Medical Marijuana........................71 Church ..........................................65 Veterinary and Kennel Directory ..... 57 Maja’s ...............................................71 Waterville First Baptist Church -

Real Estate Tax Commitment Book
Embden Real Estate Tax Commitment Book - 14.750 06/28/2019 12:45 PM 2019 PP TAX BILLS Page 1 Account Name & Address Land Building Exemption Assessment Tax 807 880 EAST SHORE REALTY 146,500 219,200 0 365,700 5,394.08 TRUST EDWARD BAUMAN, TRUSTEE Acres 1.07 5 MILK STREET, SUITE 3 Soft: 0.00 0 2,697.04 (1) PORTLAND ME 04101-4170 Mixed: 0.00 0 2,697.04 (2) Hard: 0.00 0 880 EAST SHORE ROAD 016-018 B4396P226 05/20/2011 B3302P354 05/06/2004 905 ABDELRIHIM, MOHAMED A 19,000 27,300 0 46,300 682.93 ABDELRIHIM, RHONDA L. Acres 1.00 38 ASH STREET Soft: 0.00 0 341.47 (1) Mixed: 0.00 0 341.46 (2) MADISON ME 04950 Hard: 0.00 0 558 KENNEBEC RIVER ROAD 006-054-001 B4234P180 09/16/2009 B1825P92 09/25/1992 4 ADAMS, CLAY A 1,400 0 0 1,400 20.65 92 DUTTON HILL Acres 12.70 Soft: 12.70 1,435 10.33 (1) GRAY ME 04039 Mixed: 0.00 0 10.32 (2) Hard: 0.00 0 012-019 B4714P28 09/11/2013 B2168P106 12/29/1995 B1827P14 09/29/1992 1385 ADAMS, CLAY A 24,100 90,000 0 114,100 1,682.98 92 DUTTON HILL Acres 30.32 Soft: 13.50 1,526 841.49 (1) GRAY ME 04039 Mixed: 14.80 2,324 841.49 (2) Hard: 0.00 0 329 BERT BERY ROAD 012-017-002 B4714P28 09/11/2013 B4709P95 B2791P263 204 ADAMS, SHERRI L 45,100 155,000 20,000 180,100 2,656.48 PO BOX 546 Acres 31.28 01 Homestead Exempt Soft: 0.00 0 1,328.24 (1) NORTH ANSON ME 04958 Mixed: 0.00 0 1,328.24 (2) Hard: 0.00 0 388 DUNBAR HILL ROAD 008-020 B3232P163 11/01/2003 258 ADLER, LAURA-JEAN 95,300 32,000 0 127,300 1,877.68 PO BOX 123 Acres 1.04 Soft: 0.00 0 938.84 (1) CENTERPORT NY 11721 Mixed: 0.00 0 938.84 (2) Hard: 0.00 0 852 EAST SHORE ROAD -

Real Estate Tax Commitment Book
Belgrade Real Estate Tax Commitment Book - 15.380 07/16/2021 8:13 AM 2021 TAX COMMITMENT Page 1 Account Name & Address Land Building Exemption Assessment Tax 6 176 AUGUSTA ROAD 56,800 274,900 0 331,700 5,101.55 APARTMENTS LLC PO BOX 275 Acres 5.58 AUGUSTA ME 04332 AUGUSTA ROAD 11-016D B13456P17 01/16/2020 1806 176 AUGUSTA ROAD 57,700 163,900 0 221,600 3,408.21 APARTMENTS LLC PO BOX 275 Acres 6.50 AUGUSTA ME 04332 176 AUGUSTA ROAD 11-016B B13456P17 01/16/2020 1674 2 G'S TRANSPORTATION 12,800 0 0 12,800 196.86 INC 862 WEST ROAD Acres 6.70 BELGRADE ME 04917 CASTLE ISLAND ROAD 08-010-010 B11669P306 04/22/2014 B11062P328 06/08/2012 2264 2 G's TRANSPORTATION 12,200 0 0 12,200 187.64 INC 862 WEST ROAD Acres 5.20 BELGRADE ME 04917 CASTLE ISLAND ROAD 08-010-009 B12067P117 08/07/2015 B3314P45 2344 2 G'S TRANSPORTATION 12,800 0 0 12,800 196.86 INC 862 WEST ROAD Acres 7.50 BELGRADE ME 04917 48 CASTLE ISLAND ROAD 09-011 B8645P89 06/14/2006 B4175P226 24 20 LONG POND LLC 214,500 77,900 0 292,400 4,497.11 3915 PONTE VEDRA BLVD Acres 0.65 JACKSONVILLE FL 32250 20 LONG POND DRIVE 25-045 B12572P107 Land Building Exempt Total Tax Page Totals: 366,800 516,700 0 883,500 13,588.23 Subtotals: 366,800 516,700 0 883,500 13,588.23 Belgrade Real Estate Tax Commitment Book - 15.380 07/16/2021 8:13 AM 2021 TAX COMMITMENT Page 2 Account Name & Address Land Building Exemption Assessment Tax 1573 312 TIMBERPOINT ROAD 189,900 255,900 0 445,800 6,856.40 TRUST CO-TRUSTEES: LYNN Acres 0.86 BUTLER, DAVID BUTLER 312 TIMBERPOINT ROAD BELGRADE ME 04917 312 TIMBER POINT ROAD 8A-010 -

Real Estate Tax Commitment Book
Embden Real Estate Tax Commitment Book - 12.960 07/01/2015 11:03 AM 2015 Tax Commitment Page 1 Account Name & Address Land Building Exemption Assessment Tax 807 880 EAST SHORE REALTY 146,500 224,800 0 371,300 4,812.05 TRUST BAUMANN, EDWARD Acres 1.07 (TRUSTEE) 5 MILK STREET Soft: 0.00 0 2,406.03 (1) SUITE 3 Mixed: 0.00 0 2,406.02 (2) PORTLAND ME 04101-4170 Hard: 0.00 0 880 EAST SHORE ROAD 016-018 B4396P226 05/20/2011 B3302P354 05/06/2004 Land Building Exempt Total Tax Page Totals: 146,500 224,800 0 371,300 4,812.05 Subtotals: 146,500 224,800 0 371,300 4,812.05 Embden Real Estate Tax Commitment Book - 12.960 07/01/2015 11:03 AM 2015 Tax Commitment Page 2 Account Name & Address Land Building Exemption Assessment Tax 2 ABBOTT, MARK 19,700 3,600 0 23,300 301.97 PO BOX 56 Acres 1.47 READFIELD ME 04355 Soft: 0.00 0 150.99 (1) Mixed: 0.00 0 150.98 (2) Hard: 0.00 0 429 KENNEBEC RIVER ROAD 006-040 B2784P299 04/19/2001 B1235P73 01/02/1986 905 ABDELRIHIM, MOHAMED A. 19,000 27,800 0 46,800 606.53 & RHONDA L. 38 ASH STREET Acres 1.00 MADISON ME 04950 Soft: 0.00 0 303.27 (1) Mixed: 0.00 0 303.26 (2) Hard: 0.00 0 558 KENNEBEC RIVER ROAD 006-054-001 B4234P180 09/16/2009 B1825P92 09/25/1992 1493 ADAMS, ANDREA L. -

Threats from Afar Winter 2020 Newsletter—Volume 9 When Conserving Land Isn’T Enough
Protecting the Pierce Pond watershed for thirty-one years Threats from Afar Winter 2020 newsletter—volume 9 When conserving land isn’t enough —Jerry Bley submerged aquatic plants that, when introduced into a lake or pond, can quickly The Pierce Pond Watershed Trust expand to create dense beds and mats. has excelled in conserving land over the past 30 years, success- According to the Maine DEP, milfoil fully eliminating development infestations, “have the potential to be rights in the Pierce Pond devastating with dense mats impairing watershed. Today, our full efforts boating, fishing and swimming.” Hydrilla (an are focused on acquiring lands to invasive native to Africa, Australia, and parts greatly improve stewardship of of Asia) is a prolific weed that forms stems up the watershed. to 30 feet long which can dominate freshwater ecosystems quickly. All three can reproduce However, even when we achieve from fragments created by boats, swimmers, our objective of owning most or animals. DEP states that “the introduction watershed land, there will be of one single fragment of this plant can result many threats from afar – most in the infestation of an entire lake. Once A Variable-Leaf-Milfoil-Infested Lake in Maine notably those posed by invasive introduced into a lake, they are virtually and introduced plants and impossible to eradicate.” animals that have the potential to disrupt the watershed’s natural When asked about the vulnerability of Pierce ecosystems. To better understand Pond and other ponds in the watershed, DEP’s today’s and tomorrow’s threats, McPhedran noted that the watershed has the we spoke with two experts; following factors working in its favor that Biologist John McPhedran of the reduce the risk of invasive species infestation: Invasive Aquatic Species Program Most reports of aquatic invasives have been of the Maine Department of within the southwest quadrant of the state on Environmental Protection (Maine ponds that are far more accessible than Pierce DEP), and Assistant Regional Pond. -

Maine Guide to Fishing
JUL 2 3 1986 Sebago Lake Fishing Regulations 1 9 8 2 DAILY LIMIT ON DAILY LIMIT ON OPEN-WATER FISHING ICE FISHING * This is the only non-resident license of any kind that may be exchanged; for $11.00, it may be ex changed for a Season license. MAINE FISHING RECORDS BROOK TROUT 8 lbs. 8 oz. Chase Pond 1979 BROWN TROUT 19 lbs. 7 oz. SEBAGO LAKE 1958 LAKE TROUT (TOGUE) 31 lbs. 8 oz. Beech Hill Pond 1958 LANDLOCKED SALMON 2 2 lbs. 8 oz. SEBAGO LAKE 1907 (world’s record) SMALLMOUTH BASS 8 lbs. Thompson Lake 1970 LARGEMOUTH BASS 11 lbs. 10 oz. Moose Pond 1968 WHITE PERCH 4 lbs.10 oz. Messalonskee Lake 1949 (world’s record) CHAIN PICKEREL 6 lbs. 11 oz. Androscoggin Lake 1976 WHITEF1SH 7 lbs. 8 oz. SEBAGO LAKE 1958 C U SK 17 lbs. 8 oz. Moosehead Lake 1979 EXCERPTS FROM THE STATE BIOLOGISTS 1981 ANNUAL REPORT ON SEBAGO LAKE: “Salmon growth continues to break all records since the Fishery Division began to keep records at SEBAGO LAKE back in 1957. Salmon in 1981 averaged 23.2 inches in length and 3 pounds, 15 ounces in weight. 4-year-old salmon...averages 2 4 .4 inches long and 5V2 pounds in weight! Togue...averaging 2 6 .4 inches in length and 7 pounds, 11 ounces in weight The largest togue reported weighed 18 pounds, 14 ounces... many “old time fishermen ”,.. who have fished the lake for 5 0 years or more...could not remember when larger salmon were taken. Even some of the old angler records...on people who “trained up” from Boston to fish SEBAGO LAKE in the early 1900s, were not any more impressive than the 1981 records. -

Real Estate Tax Commitment Book
Rome Real Estate Tax Commitment Book - 10.950 06/08/2021 10:45 AM 2021 Tax Commitment Page 1 Account Name & Address Land Building Exemption Assessment Tax 158 301 MOUNTAIN DRIVE 399,400 141,300 0 540,700 5,920.67 ROME, LLC C/o PENELOPE BRANNA Acres 1.25 MANAGING MEMBER 1376 US HIGHWAY 22 MOUNTAINSIDE NJ 07092 GREAT POND 20-007 B12703P132 06/28/2017 B2252P261 608 88 GOLDEN REALTY TRUST 355,900 62,400 0 418,300 4,580.39 C/o TERRENCE D HARTMAN Acres 0.64 7 LITTLES HILL LANE GEORGETOWN MA 01833 88 GOLDEN POND LANE 24-022 B11618P248 01/10/2014 361 ABBOTT, ELDON L 37,000 110,900 0 147,900 1,619.51 ABBOTT, VERE 110 MEREDITH WAY WAYMOUTH MA 02188 BLC SEC. I 15-037 B11113P26 07/26/2012 2 ABBOTT, ELDON L 13,800 0 0 13,800 151.11 110 MEREDITH WAY WAYMOUTH MA 02188 BLC 15-070 B3065P171 547 ABBOTT, JESSE 25,700 10,800 0 36,500 399.68 BRADLEY-ABBOTT, Acres 1.10 JENNIFER P.O. BOX 547 SCARBOROUGH ME 04070 727 AUGUSTA ROAD 04-001 B12940P270 06/18/2018 3 ABBOTT, VERA 37,000 69,200 0 106,200 1,162.89 110 MEREDITH WAY WEYMOUTH MA 02188 86 ASPEN ESTS. (BLC) 15-069 B3065P169 Land Building Exempt Total Tax Page Totals: 868,800 394,600 0 1,263,400 13,834.25 Subtotals: 868,800 394,600 0 1,263,400 13,834.25 Rome Real Estate Tax Commitment Book - 10.950 06/08/2021 10:45 AM 2021 Tax Commitment Page 2 Account Name & Address Land Building Exemption Assessment Tax 4 ABRUZZESE, CHERYL A 62,200 100,700 22,500 140,400 1,537.38 557 CASTLE ISLAND Acres 6.00 01 HOMESTEAD EXEMPT ROAD ME 04963 557 CASTLE ISLAND RD.