(1924) Transfered from Arctiinae (Erebidae) to Lacturidae and Immidae (Lepidoptera) and Synonymised
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1 1 DNA Barcodes Reveal Deeply Neglected Diversity and Numerous
Page 1 of 57 1 DNA barcodes reveal deeply neglected diversity and numerous invasions of micromoths in 2 Madagascar 3 4 5 Carlos Lopez-Vaamonde1,2, Lucas Sire2, Bruno Rasmussen2, Rodolphe Rougerie3, 6 Christian Wieser4, Allaoui Ahamadi Allaoui 5, Joël Minet3, Jeremy R. deWaard6, Thibaud 7 Decaëns7, David C. Lees8 8 9 1 INRA, UR633, Zoologie Forestière, F- 45075 Orléans, France. 10 2 Institut de Recherche sur la Biologie de l’Insecte, UMR 7261 CNRS Université de Tours, UFR 11 Sciences et Techniques, Tours, France. 12 3Institut de Systématique Evolution Biodiversité (ISYEB), Muséum national d'Histoire naturelle, 13 CNRS, Sorbonne Université, EPHE, 57 rue Cuvier, CP 50, 75005 Paris, France. 14 4 Landesmuseum für Kärnten, Abteilung Zoologie, Museumgasse 2, 9020 Klagenfurt, Austria 15 5 Department of Entomology, University of Antananarivo, Antananarivo 101, Madagascar 16 6 Centre for Biodiversity Genomics, University of Guelph, 50 Stone Road E., Guelph, ON 17 N1G2W1, Canada 18 7Centre d'Ecologie Fonctionnelle et Evolutive (CEFE UMR 5175, CNRS–Université de Genome Downloaded from www.nrcresearchpress.com by UNIV GUELPH on 10/03/18 19 Montpellier–Université Paul-Valéry Montpellier–EPHE), 1919 Route de Mende, F-34293 20 Montpellier, France. 21 8Department of Life Sciences, Natural History Museum, Cromwell Road, SW7 5BD, UK. 22 23 24 Email for correspondence: [email protected] For personal use only. This Just-IN manuscript is the accepted prior to copy editing and page composition. It may differ from final official version of record. 1 Page 2 of 57 25 26 Abstract 27 Madagascar is a prime evolutionary hotspot globally, but its unique biodiversity is under threat, 28 essentially from anthropogenic disturbance. -

DNA Barcodes Reveal Deeply Neglected Diversity and Numerous Invasions of Micromoths in Madagascar
Genome DNA barcodes reveal deeply neglected diversity and numerous invasions of micromoths in Madagascar Journal: Genome Manuscript ID gen-2018-0065.R2 Manuscript Type: Article Date Submitted by the 17-Jul-2018 Author: Complete List of Authors: Lopez-Vaamonde, Carlos; Institut National de la Recherche Agronomique (INRA), ; Institut de Recherche sur la Biologie de l’Insecte (IRBI), Sire, Lucas; Institut de Recherche sur la Biologie de l’Insecte Rasmussen,Draft Bruno; Institut de Recherche sur la Biologie de l’Insecte Rougerie, Rodolphe; Institut Systématique, Evolution, Biodiversité (ISYEB), Wieser, Christian; Landesmuseum für Kärnten Ahamadi, Allaoui; University of Antananarivo, Department Entomology Minet, Joël; Institut de Systematique Evolution Biodiversite deWaard, Jeremy; Biodiversity Institute of Ontario, University of Guelph, Decaëns, Thibaud; Centre d'Ecologie Fonctionnelle et Evolutive (CEFE UMR 5175, CNRS–Université de Montpellier–Université Paul-Valéry Montpellier–EPHE), , CEFE UMR 5175 CNRS Lees, David; Natural History Museum London Keyword: Africa, invasive alien species, Lepidoptera, Malaise trap, plant pests Is the invited manuscript for consideration in a Special 7th International Barcode of Life Issue? : https://mc06.manuscriptcentral.com/genome-pubs Page 1 of 57 Genome 1 DNA barcodes reveal deeply neglected diversity and numerous invasions of micromoths in 2 Madagascar 3 4 5 Carlos Lopez-Vaamonde1,2, Lucas Sire2, Bruno Rasmussen2, Rodolphe Rougerie3, 6 Christian Wieser4, Allaoui Ahamadi Allaoui 5, Joël Minet3, Jeremy R. deWaard6, Thibaud 7 Decaëns7, David C. Lees8 8 9 1 INRA, UR633, Zoologie Forestière, F- 45075 Orléans, France. 10 2 Institut de Recherche sur la Biologie de l’Insecte, UMR 7261 CNRS Université de Tours, UFR 11 Sciences et Techniques, Tours, France. -

Acoustic Communication in the Nocturnal Lepidoptera
Chapter 6 Acoustic Communication in the Nocturnal Lepidoptera Michael D. Greenfield Abstract Pair formation in moths typically involves pheromones, but some pyra- loid and noctuoid species use sound in mating communication. The signals are generally ultrasound, broadcast by males, and function in courtship. Long-range advertisement songs also occur which exhibit high convergence with commu- nication in other acoustic species such as orthopterans and anurans. Tympanal hearing with sensitivity to ultrasound in the context of bat avoidance behavior is widespread in the Lepidoptera, and phylogenetic inference indicates that such perception preceded the evolution of song. This sequence suggests that male song originated via the sensory bias mechanism, but the trajectory by which ances- tral defensive behavior in females—negative responses to bat echolocation sig- nals—may have evolved toward positive responses to male song remains unclear. Analyses of various species offer some insight to this improbable transition, and to the general process by which signals may evolve via the sensory bias mechanism. 6.1 Introduction The acoustic world of Lepidoptera remained for humans largely unknown, and this for good reason: It takes place mostly in the middle- to high-ultrasound fre- quency range, well beyond our sensitivity range. Thus, the discovery and detailed study of acoustically communicating moths came about only with the use of electronic instruments sensitive to these sound frequencies. Such equipment was invented following the 1930s, and instruments that could be readily applied in the field were only available since the 1980s. But the application of such equipment M. D. Greenfield (*) Institut de recherche sur la biologie de l’insecte (IRBI), CNRS UMR 7261, Parc de Grandmont, Université François Rabelais de Tours, 37200 Tours, France e-mail: [email protected] B. -

Phylogeny and Evolution of Lepidoptera
EN62CH15-Mitter ARI 5 November 2016 12:1 I Review in Advance first posted online V E W E on November 16, 2016. (Changes may R S still occur before final publication online and in print.) I E N C N A D V A Phylogeny and Evolution of Lepidoptera Charles Mitter,1,∗ Donald R. Davis,2 and Michael P. Cummings3 1Department of Entomology, University of Maryland, College Park, Maryland 20742; email: [email protected] 2Department of Entomology, National Museum of Natural History, Smithsonian Institution, Washington, DC 20560 3Laboratory of Molecular Evolution, Center for Bioinformatics and Computational Biology, University of Maryland, College Park, Maryland 20742 Annu. Rev. Entomol. 2017. 62:265–83 Keywords Annu. Rev. Entomol. 2017.62. Downloaded from www.annualreviews.org The Annual Review of Entomology is online at Hexapoda, insect, systematics, classification, butterfly, moth, molecular ento.annualreviews.org systematics This article’s doi: Access provided by University of Maryland - College Park on 11/20/16. For personal use only. 10.1146/annurev-ento-031616-035125 Abstract Copyright c 2017 by Annual Reviews. Until recently, deep-level phylogeny in Lepidoptera, the largest single ra- All rights reserved diation of plant-feeding insects, was very poorly understood. Over the past ∗ Corresponding author two decades, building on a preceding era of morphological cladistic stud- ies, molecular data have yielded robust initial estimates of relationships both within and among the ∼43 superfamilies, with unsolved problems now yield- ing to much larger data sets from high-throughput sequencing. Here we summarize progress on lepidopteran phylogeny since 1975, emphasizing the superfamily level, and discuss some resulting advances in our understanding of lepidopteran evolution. -

Amphiesmeno- Ptera: the Caddisflies and Lepidoptera
CY501-C13[548-606].qxd 2/16/05 12:17 AM Page 548 quark11 27B:CY501:Chapters:Chapter-13: 13Amphiesmeno-Amphiesmenoptera: The ptera:Caddisflies The and Lepidoptera With very few exceptions the life histories of the orders Tri- from Old English traveling cadice men, who pinned bits of choptera (caddisflies)Caddisflies and Lepidoptera (moths and butter- cloth to their and coats to advertise their fabrics. A few species flies) are extremely different; the former have aquatic larvae, actually have terrestrial larvae, but even these are relegated to and the latter nearly always have terrestrial, plant-feeding wet leaf litter, so many defining features of the order concern caterpillars. Nonetheless, the close relationship of these two larval adaptations for an almost wholly aquatic lifestyle (Wig- orders hasLepidoptera essentially never been disputed and is supported gins, 1977, 1996). For example, larvae are apneustic (without by strong morphological (Kristensen, 1975, 1991), molecular spiracles) and respire through a thin, permeable cuticle, (Wheeler et al., 2001; Whiting, 2002), and paleontological evi- some of which have filamentous abdominal gills that are sim- dence. Synapomorphies linking these two orders include het- ple or intricately branched (Figure 13.3). Antennae and the erogametic females; a pair of glands on sternite V (found in tentorium of larvae are reduced, though functional signifi- Trichoptera and in basal moths); dense, long setae on the cance of these features is unknown. Larvae do not have pro- wing membrane (which are modified into scales in Lepi- legs on most abdominal segments, save for a pair of anal pro- doptera); forewing with the anal veins looping up to form a legs that have sclerotized hooks for anchoring the larva in its double “Y” configuration; larva with a fused hypopharynx case. -

Journal of the Lepidopterists' Society
124 JOURNAL OF THE LEPIDOPTERISTS' SOCIETY THE STATUS OF THE GLYPHIPTERIGIDAE AND A REASSESSMENT OF RELATIONSHIPS IN YPONOMEUTOID FAMILIES AND DITRYSIAN SUPERFAMILIESl JOHN B. HEPPNER2 Department of Entomology and Nematology, IFAS, University of Florida, Gainesville, Florida 32611 Current studies of the North American Glyphipterigidae have revealed major fundamental morphological and behavioral characters which dem onstrate that the inclusion of thc choreutid and glyphipterigid groups within a single family is untenable. The discordant characters involved have been shown in the past by other workers to be so fundamentally and evolutionarily conservative in Lepidoptera phylogeny that it is not even possible to consider the two groups to have evolved within the same superfamily, Glyphipterigid moths have long been considered of unusual interest because of apparent affinities to the Yponomeutidae and the Sesiidae, as well as to the Tortricidae. Most early workers considered them as dis tinct groups: the choreutids were placed with the tortricids and the glyphipterigids sensu stricto were placed among the tineoid moths. This segregation was rarely altered until Meyrick (1914) combined them into one family. Meyrick's classification was based largely on general facies-the two groups share a number of superficial characters-and not fundamental relationships. He also relied strongly on wing venation and did not use genitalia, internal morphology or larval characters. He formed a conglomeration of what now are no less than nine distinct families in several superfamilies, although he realized the true affinities of many of the included genera in later years. Current revisionary studies on the choreutids and glyphipterigids, using modern systematic tech niques, are revealing the true affinities of these moths. -
![(Lepidoptera: Gracillariidae: Epicephala) and Leafflower Trees (Phyllanthaceae: Phyllanthus Sensu Lato [Glochidion]) in Southeastern Polynesia](https://docslib.b-cdn.net/cover/8161/lepidoptera-gracillariidae-epicephala-and-leafflower-trees-phyllanthaceae-phyllanthus-sensu-lato-glochidion-in-southeastern-polynesia-1478161.webp)
(Lepidoptera: Gracillariidae: Epicephala) and Leafflower Trees (Phyllanthaceae: Phyllanthus Sensu Lato [Glochidion]) in Southeastern Polynesia
Coevolutionary Diversification of Leafflower Moths (Lepidoptera: Gracillariidae: Epicephala) and Leafflower Trees (Phyllanthaceae: Phyllanthus sensu lato [Glochidion]) in Southeastern Polynesia By David Howard Hembry A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Environmental Science, Policy, and Management in the Graduate Division of the University of California, Berkeley Committee in charge: Professor Rosemary Gillespie, Chair Professor Bruce Baldwin Professor Patrick O’Grady Spring 2012 1 2 Abstract Coevolution between phylogenetically distant, yet ecologically intimate taxa is widely invoked as a major process generating and organizing biodiversity on earth. Yet for many putatively coevolving clades we lack knowledge both of their evolutionary history of diversification, and the manner in which they organize themselves into patterns of interaction. This is especially true for mutualistic associations, despite the fact that mutualisms have served as models for much coevolutionary research. In this dissertation, I examine the codiversification of an obligate, reciprocally specialized pollination mutualism between leafflower moths (Lepidoptera: Gracillariidae: Epicephala) and leafflower trees (Phyllanthaceae: Phyllanthus sensu lato [Glochidion]) on the oceanic islands of southeastern Polynesia. Leafflower moths are the sole known pollinators of five clades of leafflowers (in the genus Phyllanthus s. l., including the genera Glochidion and Breynia), and thus this interaction is considered to be obligate. Female moths actively transfer pollen from male flowers to female flowers, using a haired proboscis to transfer pollen into the recessed stigmatic surface at the end of the fused stylar column. The moths then oviposit into the flowers’ ovaries, and the larva which hatches consumes a subset, but not all, of the developing fruit’s seed set. -

Moths and Butterflies
LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004MOTHS LJL©2004 LJL©2004AND BUTTERFLIES LJL©2004 LJL©2004 (LEPIDOPTERA) LJL©2004 LJL©2004 LJL©2004 FROM LJL©2004 BAHÍA LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004HONDA LJL©2004 LJL©2004 AND CANALES LJL©2004 LJL©2004 DE TIERRA LJL©2004 ISLANDLJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004(VERAGUAS, LJL©2004 LJL©2004 PANAMA LJL©2004) LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 -

Taxonomy of Microlepidoptera
KFRI Research Report No. 361 Taxonomy of Microlepidoptera George Mathew Kerala Forest Research Institute An Institution of Kerala State Council for Science, Technology and Environment (KSCSTE) Peechi – 680 653, Thrissur, Kerala, India March 2010 KFRI Research Report No. 361 Taxonomy of Microlepidoptera (Final Report of the Project KFRI/ 340/2001: All India Coordinated Project on the Taxonomy of Microlepidoptera, sponsored by the Ministry of Environment and Forests, Government of India, New Delhi) George Mathew Forest Health Division Kerala Forest Research Institute Peechi-680 653, Kerala, India March 2010 Abstract of Project Proposal Project No. KFRI/340/2001 1. Title of the project: Taxonomy of Microlepidoptera 2. Objectives: • Survey, collection, identification and preservation of Microlepidoptera • Maintenance of collections and data bank • Development of identification manuals • Training of college teachers, students and local communities in Para taxonomy. 3. Date of commencement: March 2001 4. Scheduled date of completion: June 2009 5. Project team: Principal Investigator (for Kerala part): Dr. George Mathew Research Fellow: Shri. R.S.M. Shamsudeen 6. Study area: Kerala 7. Duration of the study: 2001- 2010 8. Project budget: Rs. 2.4 lakhs/ year 9. Funding agency: Ministry of Environment and Forests, New Delhi CONTENTS Abstract 1. Introduction………………………………………………………………… 1 1.1. Classification of Microheterocera………………………………………… 1 1.2. Biology and Behavior…………………………………………………….. 19 1.3. Economic importance of Microheterocera……………………………….. 20 1.4. General External Morphology……………………………………………. 21 1.5. Taxonomic Key for Seggregating higher taxa……………………………. 26 1.6. Current status of taxonomy of the group………………………………….. 28 2. Review of Literature……………………………………………………….. 30 2.1. Contributors on Microheterocera………………………………………….. 30 2.2. Microheteocera fauna of the world………………………………………… 30 2.3. -

Moths of Trinity River National Wildlife Refuge
U.S. FishFish & & Wildlife Wildlife Service Service Moths of Trinity River National Wildlife Refuge Established in 1994, the 25,000-acre Givira arbeloides Trinity River National Wildlife Refuge Prionoxystus robiniae is a remnant of what was once a much Carpenterworm Moth larger, frequently flooded, bottomland hardwood forest. You are still able to Crambid Snout Moths (Crambidae) view vast expanses of ridge and swale Achyra rantalis floodplain features, numerous bayous, Garden Webworm Moth oxbow lakes, and cypress/tupelo swamps Aethiophysa invisalis along the Trinity River. It is one of Argyria lacteella only 14 priority-one bottomland sites Milky Urola Moth identified for protection in the Texas Carectocultus perstrialis Bottomland Protection Plan. Texas is Reed-boring Crambid Moth home to an estimated 4,000 species of Chalcoela iphitalis moths. Most of the nearly 400 species of Sooty-winged Chalcoela moths listed below were photographed Chrysendeton medicinalis around the security lights at the Refuge Bold Medicine Moth Headquarters building located adjacent Colomychus talis to a bottomland hardwood forest. Many Distinguished Colomychus more moths are not even attracted to Conchylodes ovulalis lights, so additional surveys will need Zebra Conchylodes to be conducted to document those Crambus agitatellus species. These forests also support a Double-banded Grass-veneer wide diversity of mammals, reptiles, Crambus satrapellus amphibians, and fish with many feeding Crocidophora tuberculalis on moths or their larvae. Pale-winged Crocidophora Moth Desmia funeralis For more information, visit our website: Grape leaf-folder www.fws.gov/southwest Desmia subdivisalis Diacme elealis Contact the Refuge staff if you should Paler Diacme Moth find an unlisted or rare species during Diastictis fracturalis your visit and provide a description. -
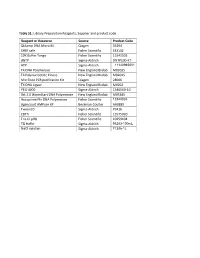
Table S1: Library Preparation Reagents, Supplier and Product Code
Table S1: Library Preparation Reagents, Supplier and product code. Reagent or Resource Source Product Code QIAamp DNA Micro Kit Qiagen 56304 SYBR safe Fisher Scientific S33102 10X Buffer Tango Fisher Scientific 11541505 dNTP Sigma-Aldrich DNTP100-KT ATP Sigma-Aldrich 11140965001 T4 DNA Polymerase New England Biolab M0203S T4 Polynucleotide Kinase New England Biolab M0201S Min Elute PCR purification Kit Qiagen 28006 T4 DNA Ligase New England Biolab M0202 PEG-4000 Sigma-Aldrich 1546569-1G Bst 2.0 WarmStart DNA Polymerase New England Biolab M0538S Accuprime Pfx DNA Polymerase Fisher Scientific 12344024 Agencourt AMPure XP Beckman Coulter A63880 Tween20 Sigma-Aldrich P9416 EDTA Fisher Scientific 15575020 Tris-Cl pH8 Fisher Scientific 10259194 TE buffer Sigma-Aldrich 93283-100mL NaCl solution Sigma-Aldrich 71386-1L Table S2: Complete list of taxa included in the analysis Code Superfamily Family Genus Species NCBI accession Type of data NW_T143 Bombycoidea Apatelodidae Apatelodes pithala SRR1794084 Transcriptome NW_G003 Bombycoidea Bombycidae Bombyx mori GCF_000151625.1 Genome NW_T076 Bombycoidea Saturniidae Actias luna SRR1002974 Transcriptome NW_T134 Bombycoidea Saturniidae Antheraea yamamai SRR1743839 Transcriptome NW_T195 Bombycoidea Saturniidae Attacus atlas SRR1002994 Transcriptome NW_T077 Bombycoidea Saturniidae Eacles imperialis SRR1299435 Transcriptome NW_T131 Bombycoidea Saturniidae Rhodinia newara SRR1743843 Transcriptome NW_T130 Bombycoidea Saturniidae Samia ricini SRR1743841 Transcriptome NW_T078 Bombycoidea Saturniidae Therinia -

A Molecular Phylogeny for Yponomeutoidea (Insecta, Lepidoptera, Ditrysia) and Its Implications for Classification, Biogeography and the Evolution of Host Plant Use
A Molecular Phylogeny for Yponomeutoidea (Insecta, Lepidoptera, Ditrysia) and Its Implications for Classification, Biogeography and the Evolution of Host Plant Use Jae-Cheon Sohn1*, Jerome C. Regier1, Charles Mitter1, Donald Davis2, Jean-Franc¸ois Landry3, Andreas Zwick4, Michael P. Cummings5 1 Department of Entomology, University of Maryland, College Park, Maryland, United States of America, 2 Department of Entomology, National Museum of Natural History, Smithsonian Institution, Washington DC, United States of America, 3 Agriculture and Agri-Food Canada, Eastern Cereal and Oilseed Research Centre, C.E.F., Ottawa, Canada, 4 Department of Entomology, State Museum of Natural History, Stuttgart, Germany, 5 Laboratory of Molecular Evolution, Center for Bioinformatics and Computational Biology, University of Maryland, College Park, Maryland, United States of America Abstract Background: Yponomeutoidea, one of the early-diverging lineages of ditrysian Lepidoptera, comprise about 1,800 species worldwide, including notable pests and insect-plant interaction models. Yponomeutoids were one of the earliest lepidopteran clades to evolve external feeding and to extensively colonize herbaceous angiosperms. Despite the group’s economic importance, and its value for tracing early lepidopteran evolution, the biodiversity and phylogeny of Yponomeutoidea have been relatively little studied. Methodology/Principal Findings: Eight nuclear genes (8 kb) were initially sequenced for 86 putative yponomeutoid species, spanning all previously recognized suprageneric groups, and 53 outgroups representing 22 families and 12 superfamilies. Eleven to 19 additional genes, yielding a total of 14.8 to 18.9 kb, were then sampled for a subset of taxa, including 28 yponomeutoids and 43 outgroups. Maximum likelihood analyses were conducted on data sets differing in numbers of genes, matrix completeness, inclusion/weighting of synonymous substitutions, and inclusion/exclusion of ‘‘rogue’’ taxa.