Therapeutic Opportunities Through Modulation of the Endocannabinoid System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Journal of Biological Rhythms Official Publication of the Society for Research on Biological Rhythms
Journal of Biological Rhythms Official Publication of the Society for Research on Biological Rhythms Volume 16, Issue 6 December 2001 EDITORIAL Pebbles of Truth 515 Martin Zatz FEATURE Review Clockless Yeast and the Gears of the Clock: How Do They Mesh? 516 Ruben Baler ARTICLES Resetting of the Circadian Clock by Phytochromes 523 and Cryptochromes in Arabidopsis Marcelo J. Yanovsky, M. Agustina Mazzella, Garry C. Whitelam, and Jorge J. Casal Distinct Pharmacological Mechanisms Leading to c-fos 531 Gene Expression in the Fetal Suprachiasmatic Nucleus Lauren P. Shearman and David R. Weaver Daily Novel Wheel Running Reorganizes and 541 Splits Hamster Circadian Activity Rhythms Michael R. Gorman and Theresa M. Lee Temporal Reorganization of the Suprachiasmatic Nuclei 552 in Hamsters with Split Circadian Rhythms Michael R. Gorman, Steven M. Yellon, and Theresa M. Lee Light-Induced Resetting of the Circadian Pacemaker: 564 Quantitative Analysis of Transient versus Steady-State Phase Shifts Kazuto Watanabe, Tom Deboer, and Johanna H. Meijer Temperature Cycles Induce a Bimodal Activity Pattern in Ruin Lizards: 574 Masking or Clock-Controlled Event? A Seasonal Problem Augusto Foà and Cristiano Bertolucci LETTER Persistence of Masking Responses to Light in Mice Lacking Rods and Cones 585 N. Mrosovsky, Robert J. Lucas, and Russell G. Foster MEETING Eighth Meeting of the Society for Research on Biological Rythms 588 Index 589 Journal of Biological Rhythms Official Publication of the Society for Research on Biological Rhythms EDITOR-IN-CHIEF Martin Zatz FEATURES EDITORS ASSOCIATE EDITORS Larry Morin Michael Hastings SUNY, Stony Brook University of Cambridge Anna Wirz-Justice Ken-Ichi Honma University of Basel Hokkaido Univ School Medicine Michael Young Rockefeller University EDITORIAL BOARD Josephine Arendt Terry Page University of Surrey Vanderbilt University Charles A. -

A Review of Effective ADHD Treatment Devin Hilla Grand Valley State University, [email protected]
Grand Valley State University ScholarWorks@GVSU Honors Projects Undergraduate Research and Creative Practice 12-2015 Changing Behavior, Brain Differences, or Both? A Review of Effective ADHD Treatment Devin Hilla Grand Valley State University, [email protected] Follow this and additional works at: http://scholarworks.gvsu.edu/honorsprojects Part of the Medicine and Health Sciences Commons Recommended Citation Hilla, Devin, "Changing Behavior, Brain Differences, or Both? A Review of Effective ADHD Treatment" (2015). Honors Projects. 570. http://scholarworks.gvsu.edu/honorsprojects/570 This Open Access is brought to you for free and open access by the Undergraduate Research and Creative Practice at ScholarWorks@GVSU. It has been accepted for inclusion in Honors Projects by an authorized administrator of ScholarWorks@GVSU. For more information, please contact [email protected]. Running Head: EFFECTIVE ADHD TREATMENT 1 Changing Behavior, Brain Differences, or Both? A Review of Effective ADHD Treatment Devin Hilla Grand Valley State University Honors Senior Thesis EFFECTIVE ADHD TREATMENT 2 Abstract Much debate exists over the proper course of treatment for individuals with attention- deficit/hyperactivity disorder (ADHD). Stimulant medications, such as methylphenidate (e.g., Ritalin) and amphetamine (e.g., Adderall), have been shown to be effective in managing ADHD symptoms. More recently, non-stimulant medications, such as atomoxetine (e.g., Strattera), clonidine (e.g., Kapvay), and guanfacine (e.g., Intuniv), have provided a pharmacological alternative with potentially lesser side effects than stimulants. Behavioral therapies, like behavioral parent training, behavioral classroom management, and behavioral peer interventions, have shown long-term benefits for children with ADHD; however, the success of the short-term management of ADHD symptoms is not as substantial when compared with stimulant medications. -

Gabab Regulation of Methamphetamine-Induced Associative Learning
Loyola University Chicago Loyola eCommons Dissertations Theses and Dissertations 2010 Gabab Regulation of Methamphetamine-Induced Associative Learning Robin Michelle Voigt Loyola University Chicago Follow this and additional works at: https://ecommons.luc.edu/luc_diss Part of the Pharmacology Commons Recommended Citation Voigt, Robin Michelle, "Gabab Regulation of Methamphetamine-Induced Associative Learning" (2010). Dissertations. 38. https://ecommons.luc.edu/luc_diss/38 This Dissertation is brought to you for free and open access by the Theses and Dissertations at Loyola eCommons. It has been accepted for inclusion in Dissertations by an authorized administrator of Loyola eCommons. For more information, please contact [email protected]. This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 3.0 License. Copyright © 2010 Robin Michelle Voigt LOYOLA UNIVERSITY CHICAGO GABAB REGULATION OF METHAMPHETAMINE-INDUCED ASSOCIATIVE LEARNING A DISSERTATION SUBMITTED TO THE FACULTY OF THE GRADUATE SCHOOL IN CANDIDACY FOR THE DEGREE OF DOCTOR OF PHILOSOPHY PROGRAM IN MOLECULAR PHARMACOLOGY & THERAPEUTICS BY ROBIN MICHELLE VOIGT CHICAGO, IL DECEMBER 2010 Copyright by Robin Michelle Voigt, 2010 All rights reserved ACKNOWLEDGEMENTS Without the support of so many generous and wonderful individuals I would not have been able to be where I am today. First, I would like to thank my Mother for her belief that I could accomplish anything that I set my mind to. I would also like to thank my dissertation advisor, Dr. Celeste Napier, for encouraging and challenging me to be better than I thought possible. I extend gratitude to my committee members, Drs. Julie Kauer, Adriano Marchese, Micky Marinelli, and Karie Scrogin for their guidance and insightful input. -

And Anxiety-Related Behavioral Effects of Repeated Nicotine As a Stressor: the Role of Cannabinoid Receptors Tamaki Hayase
Hayase BMC Neuroscience 2013, 14:20 http://www.biomedcentral.com/1471-2202/14/20 RESEARCH ARTICLE Open Access Working memory- and anxiety-related behavioral effects of repeated nicotine as a stressor: the role of cannabinoid receptors Tamaki Hayase Abstract Background: Like emotional symptoms such as anxiety, modulations in working memory are among the frequently-reported but controversial psychiatric symptoms associated with nicotine (NC) administration. In the present study, repeated NC-induced modulations in working memory, along with concurrently-observed anxiety- related behavioral alterations, were investigated in mice, and compared with the effects of a typical cognition- impairing stressor, immobilization stress (IM). Furthermore, considering the structural and functional contributions of brain cannabinoid (CB) receptors in NC-induced psychiatric symptoms including emotional symptoms, the interactive effects of brain CB receptor ligands (CB ligands) and NC and/or IM on the working memory- and anxiety-related behaviors were examined. Results: Statistically significant working memory impairment-like behavioral alterations in the Y-maze test and anxiety-like behavioral alterations in the elevated plus-maze (EPM) test were observed in the groups of mice treated with 0.8 mg/kg NC (subcutaneous (s.c.) 0.8 mg/kg treatment, 4 days) and/or IM (10 min treatment, 4 days). In the group of mice treated with NC plus IM (NC-IM group), an enhancement of the behavioral alterations was observed. Among the CB type 1 (CB1) antagonist AM 251 (AM), the non-selective CB agonist CP 55,940 (CP), and the CB1 partial agonist/antagonist virodhamine (VD), significant recovering effects were provided by AM (0.2-2.5 mg/kg) and VD (5 mg/kg) against the working memory impairment-like behaviors, whereas significant anxiolytic-like effects (recoveries from both attenuated percentage of entries into open arms and attenuated percentage of time spent on open arms) were provided by VD (1–10 mg/kg) and CP (2 mg/kg) against the anxiety-like behaviors. -

Cannabinoids in Dermatology: a Scoping Review
Volume 24 Number 6| June 2018| Dermatology Online Journal || Review 24(6): 1 Cannabinoids in dermatology: a scoping review Lauren R M Eagelston1 MD, Nazanin Kuseh Kalani Yazd1 BS, Ravi R Patel2 MD, Hania K Flaten1 BS, Cory A Dunnick3,4 MD, Robert P Dellavalle3,4 MD PhD MSPH Affiliations: 1University of Colorado School of Medicine, Aurora, Colorado, USA, 2Gwinnett Medical Center, Lawrenceville, Georgia, USA, 3University of Colorado School of Medicine, Department of Dermatology, Aurora, Colorado, 4Denver Veterans Affairs Medical Center (VAMC), Department of Dermatology, Denver, Colorado Corresponding Author: Robert P. Dellavalle MD PhD MSPH, Professor of Dermatology, Chief of Dermatology Service, Department of Veteran Affairs Medical Center, 1055 Clermont Rm 6A-105 Mail Stop 16, Denver, CO 80220, Tel: 303.339.8020 x2475, Email: [email protected] Introduction Abstract The cannabinoids are a diverse class of The therapeutic applications of cannabis and pharmacologically active compounds that are cannabinoids are an increasingly conspicuous topic structurally and biologically similar to the primary as de-criminalization and legalization of these products continues to expand. A limited number of psychoactive compound derived from Cannabis cannabinoid compounds have been approved for a sativa -tetrahydrocannabinol (THC), [1-4]. There specific set of conditions. However, the current role are three classes of cannabinoids. Endogenous of cannabinoids for the treatment of dermatologic cannabinoids (endocannabinoids) occur conditions remains to be defined. We conducted a naturally/are constitutively produced in the bodies review of the current literature to determine the of humans and animals [3, 5, 6]. The most well-known applications of cannabinoids for the therapy of members of this class include 2-arachidonoyl- various skin diseases. -

Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System
International Journal of Molecular Sciences Review Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System Shenglong Zou and Ujendra Kumar * Faculty of Pharmaceutical Sciences, The University of British Columbia, Vancouver, BC V6T 1Z4, Canada; [email protected] * Correspondence: [email protected]; Tel.: +1-604-827-3660; Fax: +1-604-822-3035 Received: 9 February 2018; Accepted: 11 March 2018; Published: 13 March 2018 Abstract: The biological effects of cannabinoids, the major constituents of the ancient medicinal plant Cannabis sativa (marijuana) are mediated by two members of the G-protein coupled receptor family, cannabinoid receptors 1 (CB1R) and 2. The CB1R is the prominent subtype in the central nervous system (CNS) and has drawn great attention as a potential therapeutic avenue in several pathological conditions, including neuropsychological disorders and neurodegenerative diseases. Furthermore, cannabinoids also modulate signal transduction pathways and exert profound effects at peripheral sites. Although cannabinoids have therapeutic potential, their psychoactive effects have largely limited their use in clinical practice. In this review, we briefly summarized our knowledge of cannabinoids and the endocannabinoid system, focusing on the CB1R and the CNS, with emphasis on recent breakthroughs in the field. We aim to define several potential roles of cannabinoid receptors in the modulation of signaling pathways and in association with several pathophysiological conditions. We believe that the therapeutic significance of cannabinoids is masked by the adverse effects and here alternative strategies are discussed to take therapeutic advantage of cannabinoids. Keywords: cannabinoid; endocannabinoid; receptor; signaling; central nervous system 1. Introduction The plant Cannabis sativa, better known as marijuana, has long been used for medical purpose throughout human history. -

N-Acyl-Dopamines: Novel Synthetic CB1 Cannabinoid-Receptor Ligands
Biochem. J. (2000) 351, 817–824 (Printed in Great Britain) 817 N-acyl-dopamines: novel synthetic CB1 cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity in vitro and in vivo Tiziana BISOGNO*, Dominique MELCK*, Mikhail Yu. BOBROV†, Natalia M. GRETSKAYA†, Vladimir V. BEZUGLOV†, Luciano DE PETROCELLIS‡ and Vincenzo DI MARZO*1 *Istituto per la Chimica di Molecole di Interesse Biologico, C.N.R., Via Toiano 6, 80072 Arco Felice, Napoli, Italy, †Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry, R. A. S., 16/10 Miklukho-Maklaya Str., 117871 Moscow GSP7, Russia, and ‡Istituto di Cibernetica, C.N.R., Via Toiano 6, 80072 Arco Felice, Napoli, Italy We reported previously that synthetic amides of polyunsaturated selectivity for the anandamide transporter over FAAH. AA-DA fatty acids with bioactive amines can result in substances that (0.1–10 µM) did not displace D1 and D2 dopamine-receptor interact with proteins of the endogenous cannabinoid system high-affinity ligands from rat brain membranes, thus suggesting (ECS). Here we synthesized a series of N-acyl-dopamines that this compound has little affinity for these receptors. AA-DA (NADAs) and studied their effects on the anandamide membrane was more potent and efficacious than anandamide as a CB" transporter, the anandamide amidohydrolase (fatty acid amide agonist, as assessed by measuring the stimulatory effect on intra- hydrolase, FAAH) and the two cannabinoid receptor subtypes, cellular Ca#+ mobilization in undifferentiated N18TG2 neuro- CB" and CB#. NADAs competitively inhibited FAAH from blastoma cells. This effect of AA-DA was counteracted by the l µ N18TG2 cells (IC&! 19–100 M), as well as the binding of the CB" antagonist SR141716A. -

The Cannabinoid Receptor Agonist WIN 55,212-2 Attenuates the Effects Induced by Quinolinic Acid in the Rat Striatum
Neuropharmacology 51 (2006) 1004e1012 www.elsevier.com/locate/neuropharm The cannabinoid receptor agonist WIN 55,212-2 attenuates the effects induced by quinolinic acid in the rat striatum A. Pintor a, M.T. Tebano a, A. Martire a, R. Grieco a, M. Galluzzo a, M.L. Scattoni b,A.Pe`zzola a, R. Coccurello c, F. Felici a, V. Cuomo d, D. Piomelli e, G. Calamandrei b, P. Popoli a,* a Department of Drug Research and Evaluation, Central Nervous System Pharmacology Division, Istituto Superiore di Sanita`, Viale Regina Elena, 299, 00161 Rome, Italy b Department of Cell Biology and Neuroscience, Istituto Superiore di Sanita`, Viale Regina Elena, 299, 00161 Rome, Italy c Institute of Neuroscience, EBRI Foundation, Rome, Italy d Department of Pharmacology and General Physiology, University ‘‘La Sapienza’’, Rome, Italy e Department of Pharmacology and Center for Drug Discovery, University of California, Irvine, CA, USA Received 7 April 2006; received in revised form 15 May 2006; accepted 16 June 2006 Abstract The ability of CB1 receptors to regulate the release of glutamate in the striatum, together with the finding that, in experimental models of Huntington disease (HD), both endocannabinoid levels and CB1 receptor densities are reduced, has prompted the investigation on the neuropro- tective role of the cannabinoids in HD. Quinolinic acid (QA) is an excitotoxin that, when injected in the rat striatum reproduces many features of HD and that acts by stimulating glutamate outflow. The aim of the present study was to test the ability of the cannabinoid receptor agonist WIN 55,212-2 to prevent the effects induced by QA in the rat striatum. -

The Endogenous Cannabinoid 2-Arachidonoylglycerol Is Intravenously Self-Administered by Squirrel Monkeys
The Journal of Neuroscience, May 11, 2011 • 31(19):7043–7048 • 7043 Brief Communications The Endogenous Cannabinoid 2-Arachidonoylglycerol Is Intravenously Self-Administered by Squirrel Monkeys Zuzana Justinova´,1,2 Sevil Yasar,3 Godfrey H. Redhi,1 and Steven R. Goldberg1 1Preclinical Pharmacology Section, Behavioral Neuroscience Research Branch, Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, Department of Health and Human Services, Baltimore, Maryland 21224, 2Maryland Psychiatric Research Centre, Department of Psychiatry, University of Maryland School of Medicine, Baltimore, Maryland 21228, and 3Division of Geriatric Medicine and Gerontology, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland 21224 Two endogenous ligands for cannabinoid CB1 receptors, anandamide (N-arachidonoylethanolamine) and 2-arachidonoylglycerol (2-AG), have been identified and characterized. 2-AG is the most prevalent endogenous cannabinoid ligand in the brain, and electrophysiological studies suggest 2-AG, rather than anandamide, is the true natural ligand for cannabinoid receptors and the key endocannabinoid involved in retrograde signaling in the brain. Here, we evaluated intravenously administered 2-AG for reinforcing effects in nonhuman primates. Squirrel monkeys that previously self-administered anandamide or nicotine under a fixed-ratio schedule with a 60 s timeout after each injection had their self-administration behavior extinguished by vehicle substitution and were then given the opportunity to self-administer 2-AG. Intravenous 2-AG was a very effective reinforcer of drug-taking behavior, maintaining higher numbers of self-administered injections per session and higher rates of responding than vehicle across a wide range of doses. To assess involvement of CB1 receptors in the reinforcing effects of 2-AG, we pretreated monkeys with the cannabinoid CB1 receptor inverse agonist/antagonist rimonabant [N-piperidino-5-(4-chlorophenyl)-1-(2,4- dichlorophenyl)-4-methylpyrazole-3-carboxamide]. -

Role of the Endocannabinoid System and Medical Cannabis
Brigham Young University BYU ScholarsArchive Student Works 2016-12-19 Role of the Endocannabinoid System and Medical Cannabis Sabrina Jarvis Brigham Young University, [email protected] Sean Rasmussen Brigham Young University, [email protected] Blaine Winters Brigham Young University - Provo Follow this and additional works at: https://scholarsarchive.byu.edu/studentpub Part of the Nursing Commons The College of Nursing showcases some of our best evidence based scholarly papers from graduate students in the Family Nurse Practitioner Program. The papers address relevant clinical problems for advance practice nurses and are based on the best evidence available. Using a systematic approach students critically analyze and synthesize the research studies to determine the strength of the evidence regarding the clinical problem. Based on the findings, recommendations are made for clinical practice. The papers are published in professional journals and presented at professional meetings. BYU ScholarsArchive Citation Jarvis, Sabrina; Rasmussen, Sean; and Winters, Blaine, "Role of the Endocannabinoid System and Medical Cannabis" (2016). Student Works. 192. https://scholarsarchive.byu.edu/studentpub/192 This Master's Project is brought to you for free and open access by BYU ScholarsArchive. It has been accepted for inclusion in Student Works by an authorized administrator of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. Role of the Endocannabinoid System and Medical Cannabis Sean I. Rasmussen An evidence based scholarly paper submitted to the faculty of Brigham Young University in partial fulfillment of the requirements for the degree of Masters of Science Sabrina Jarvis, Chair Blaine Winters College of Nursing Brigham Young University Copyright © 2016 Sean I. -
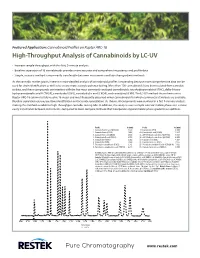
High-Throughput Analysis of Cannabinoids by LC-UV
Featured Application: Cannabinoid Profiles on Raptor ARC-18 High-Throughput Analysis of Cannabinoids by LC-UV • Increase sample throughput with this fast, 9-minute analysis. • Baseline separation of 16 cannabinoids provides more accurate and comprehensive potency and profile data. • Simple, isocratic method is more easily transferable between instruments and labs than gradient methods. As the cannabis market grows, interest in more detailed analysis of cannabinoid profiles is expanding because more comprehensive data can be used for strain identification as well as to ensure more accurate potency testing. More than 100 cannabinoids have been isolated from cannabis to date, and these compounds can interfere with the five most commonly analyzed cannabinoids: tetrahydrocannabinol (THC), delta-9-tetra- hydrocannabinolic acid A (THCA), cannabidiol (CBD), cannabidiolic acid (CBDA), and cannabinol (CBN). The LC-UV method shown here uses a Raptor ARC-18 column to fully resolve 16 major and most frequently observed minor cannabinoids for which commercial standards are available. Baseline separation ensures positive identification and accurate quantitation. As shown, all compounds were resolved in a fast 9-minute analysis, making this method suitable for high-throughput cannabis testing labs. In addition, this analysis uses a simple isocratic mobile phase so it is more easily transferable between instruments, compared to more complex methods that incorporate atypical mobile phase gradients or additives. Peaks tR (min) Peaks tR (min) 1. Cannabidivarinic acid (CBDVA) 1.877 9. Cannabinol (CBN) 4.609 2. Cannabidivarin (CBDV) 2.086 10. Cannabinolic acid (CBNA) 5.437 3. Cannabidiolic acid (CBDA) 2.592 11. Δ9-Tetrahydrocannabinol (Δ9-THC) 5.815 4. Cannabigerolic acid (CBGA) 2.750 12. -

Efficacy of Cannabinoids in a Pre-Clinical Drug-Screening Platform for Alzheimer’S Disease
Molecular Neurobiology https://doi.org/10.1007/s12035-019-1637-8 Efficacy of Cannabinoids in a Pre-Clinical Drug-Screening Platform for Alzheimer’s Disease David Schubert1 & Devin Kepchia1 & Zhibin Liang1 & Richard Dargusch1 & Joshua Goldberg & Pamela Maher1 Received: 30 January 2019 /Accepted: 6 May 2019 # Springer Science+Business Media, LLC, part of Springer Nature 2019 Abstract Finding a therapy for Alzheimer’s disease (AD) is perhaps the greatest challenge for modern medicine. The chemical scaffolds of many drugs in the clinic today are based upon natural products from plants, yet Cannabis has not been extensively examined as a source of potential AD drug candidates. Here, we determine if a number of non-psychoactive cannabinoids are neuroprotective in a novel pre-clinical AD and neurodegeneration drug-screening platform that is based upon toxicities associated with the aging brain. This drug discovery paradigm has yielded several compounds in or approaching clinical trials for AD. Eleven cannabinoids were assayed for neuroprotection in assays that recapitulate proteotoxicity, loss of trophic support, oxidative stress, energy loss, and inflammation. These compounds were also assayed for their ability to remove intraneuronal amyloid and subjected to a structure-activity relationship analysis. Pairwise combinations were assayed for their ability to synergize to produce neuropro- tective effects that were greater than additive. Nine of the 11 cannabinoids have the ability to protect cells in four distinct phenotypic neurodegeneration screening assays, including those using neurons that lack CB1 and CB2 receptors. They are able to remove intraneuronal Aβ, reduce oxidative damage, and protect from the loss of energy or trophic support.