Peptide and Protein Pegylation: a Review of Problems and Solutions Francesco M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

COVID-19 Vaccines: Update on Allergic Reactions, Contraindications, and Precautions
Centers for Disease Control and Prevention Center for Preparedness and Response COVID-19 Vaccines: Update on Allergic Reactions, Contraindications, and Precautions Clinician Outreach and Communication Activity (COCA) Webinar Wednesday, December 30, 2020 Continuing Education Continuing education will not be offered for this COCA Call. To Ask a Question ▪ All participants joining us today are in listen-only mode. ▪ Using the Webinar System – Click the “Q&A” button. – Type your question in the “Q&A” box. – Submit your question. ▪ The video recording of this COCA Call will be posted at https://emergency.cdc.gov/coca/calls/2020/callinfo_123020.asp and available to view on-demand a few hours after the call ends. ▪ If you are a patient, please refer your questions to your healthcare provider. ▪ For media questions, please contact CDC Media Relations at 404-639-3286, or send an email to [email protected]. Centers for Disease Control and Prevention Center for Preparedness and Response Today’s First Presenter Tom Shimabukuro, MD, MPH, MBA CAPT, U.S. Public Health Service Vaccine Safety Team Lead COVID-19 Response Centers for Disease Control and Prevention Centers for Disease Control and Prevention Center for Preparedness and Response Today’s Second Presenter Sarah Mbaeyi, MD, MPH CDR, U.S. Public Health Service Clinical Guidelines Team COVID-19 Response Centers for Disease Control and Prevention National Center for Immunization & Respiratory Diseases Anaphylaxis following mRNA COVID-19 vaccination Tom Shimabukuro, MD, MPH, MBA CDC COVID-19 Vaccine -

The Total Synthesis of Securinine and Other Methodology Studies
University of Windsor Scholarship at UWindsor Electronic Theses and Dissertations Theses, Dissertations, and Major Papers 2010 The total synthesis of securinine and other methodology studies Bhartesh Dhudshia University of Windsor Follow this and additional works at: https://scholar.uwindsor.ca/etd Recommended Citation Dhudshia, Bhartesh, "The total synthesis of securinine and other methodology studies" (2010). Electronic Theses and Dissertations. 8275. https://scholar.uwindsor.ca/etd/8275 This online database contains the full-text of PhD dissertations and Masters’ theses of University of Windsor students from 1954 forward. These documents are made available for personal study and research purposes only, in accordance with the Canadian Copyright Act and the Creative Commons license—CC BY-NC-ND (Attribution, Non-Commercial, No Derivative Works). Under this license, works must always be attributed to the copyright holder (original author), cannot be used for any commercial purposes, and may not be altered. Any other use would require the permission of the copyright holder. Students may inquire about withdrawing their dissertation and/or thesis from this database. For additional inquiries, please contact the repository administrator via email ([email protected]) or by telephone at 519-253-3000ext. 3208. The Total Synthesis of Securinine and Other Methodology Studies by Bhartesh Dhudshia A Dissertation Submitted to the Faculty of Graduate Studies through the Department of Chemistry and Biochemistry in Partial Fulfillment of the Requirements -

Therapeutic Oligos & Peptides
Focus on Therapeutic Oligos & Peptides Enhancing the pharmaceutical properties of peptides To begin the discussion about enhancing or improving pharmaceutical properties, one must fi rst understand “the good, the bad, and the ugly” of peptides (1). The good. Peptides are generally highly potent, selective, and have a low potential for toxicity and low risk of drug-drug interaction. The bad. Peptides are generally not terribly stable in biological matrices, susceptible to protease degradation. The ugly. The polar nature of the peptide bond and the size of peptide molecules makes permeability across cell membranes challenging. In small molecule drug PEGylation development, we commonly think PEGylation refers to the attachment about Lipinski’s rule of fi ve (2), of poly(ethylene glycol) or PEG to which is based on the observation Keyw ds peptides or proteins and is able that most orally administered drugs to improve the pharmacokinetic have common physicochemical PEGylation, lipidation, properties of these molecules. characteristics, namely, glycosylation, PEG increases the hydration shell 1. a molecular mass less than 500 cyclization, of a peptide, making the peptide daltons non-natural amino less susceptible to renal clearance 2. a logP (octanol-water partition acid substitution and protease degradation. coeffi cient) less than 5 PEGylation can also decrease the 3. no more than 5 hydrogen bond immunogenicity potential. There are donors many diff erent PEG molecules that can be covalently 4. no more than 10 (2 x 5) hydrogen bond acceptors. attached to peptides including linear or branched, low Peptides violate each and every one of these rules, molecular weight or high molecular weight. -

Site-Specific Pegylation of Therapeutic Proteins
Int. J. Mol. Sci. 2015, 16, 25831-25864; doi:10.3390/ijms161025831 OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Review Site-Specific PEGylation of Therapeutic Proteins Jonathan K. Dozier 1 and Mark D. Distefano 1,2,* 1 Department of Chemistry, University of Minnesota, Minneapolis, MN 55455, USA; E-Mail: [email protected] 2 Department of Medicinal Chemistry, University of Minnesota, Minneapolis, MN 55455, USA * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-612-624-0544; Fax: +1-612-626-7541. Academic Editor: Qiang "Shawn" Chen Received: 27 August 2015 / Accepted: 20 October 2015 / Published: 28 October 2015 Abstract: The use of proteins as therapeutics has a long history and is becoming ever more common in modern medicine. While the number of protein-based drugs is growing every year, significant problems still remain with their use. Among these problems are rapid degradation and excretion from patients, thus requiring frequent dosing, which in turn increases the chances for an immunological response as well as increasing the cost of therapy. One of the main strategies to alleviate these problems is to link a polyethylene glycol (PEG) group to the protein of interest. This process, called PEGylation, has grown dramatically in recent years resulting in several approved drugs. Installing a single PEG chain at a defined site in a protein is challenging. Recently, there is has been considerable research into various methods for the site-specific PEGylation of proteins. This review seeks to summarize that work and provide background and context for how site-specific PEGylation is performed. -

Benzyl Chloroformate (CHLOROFORMIC ACID, BENZYL ESTER)
Rev B Benzyl Chloroformate (CHLOROFORMIC ACID, BENZYL ESTER) C8H7O2Cl Molecular Weight = 170.6 CAS# 501‐53‐1 SPECIFICATIONS Assay: 98.% min. Color (APHA): 50 max. Benzyl Alcohol: 0.1% max. Hydrogen Chloride: 0.1% max. Benzyl Chloride: 1.5% max. Phosgene: 0.1% max. Dibenzyl Carbonate: 0.5% max. Iron: 1.5 PPM max. PHYSICAL PROPERTIES Appearance: Clear liquid free of visible contaminants BP: Decomposes at elevated temperature Odor: Pungent Density: 1.195 ‐1.22 MP/Range: ‐30°C Flash Point: 126°C NOTICE: The technical information and suggestions for use made herein are based on VanDeMark’s research and experience and are believed to be reliable, but such information and suggestions do not constitute a warranty, and no patent liability can be assumed. This publication is not to be taken as a license to operate under or infringe on any patents. Since VanDeMark has no control over the conditions under which the product is transported, stored, handled, used or applied, buyer must determine for himself by preliminary tests or otherwise, the suitability of the product for his purposes. VanDeMark’s liability on any basis is limited to the price of the product used. The information in this bulletin supersedes all previously issued bulletins on the subject matter covered. VanDeMark Benzyl Chloroformate APPLICATIONS SPILLS AND DISPOSAL Benzyl Chloroformate is a reactive chemical intermediate Use personal protective equipment (see MSDS). used in the synthesis of pharmaceutical and agrochemical Evacuate personnel to safe areas. Dike far ahead of products. It is used as a reagent in peptide synthesis to liquid spill for later disposal. -

Moderna COVID-19 Vaccine Vaccine Preparation and Administration Summary
Moderna COVID-19 Vaccine Vaccine Preparation and Administration Summary General Information Age Indications Vaccine: Moderna COVID-19 Vaccine 18 years of age and older Two multidose vial presentations: Schedule Maximum of 11 doses per vial 2-dose series separated by 1 month (28 days). A series started with Maximum of 15 doses per vial Moderna COVID-19 Vaccine should be completed with this product. Dosage: 0.5 mL Do NOT mix with a diluent. Administration Intramuscular (IM) injection in the deltoid muscle Thawing Frozen Vaccine Frozen vaccine must be thawed before using. Amount of time needed to thaw vaccine varies based on Thaw vaccine in the refrigerator or at room temperature: temperature and number of vials. Refrigerator: Between 2°C and 8°C (36°F and 46°F). » In the refrigerator: Up to 3 hours Unpunctured vials may be stored in the refrigerator » Room temperature: Up to 1 hour and 30 minutes for up to 30 days. Do NOT refreeze thawed vaccine. Room temperature: Between 8°C and 25°C (46°F and 77°F). Use vials in the refrigerator before removing vials from the freezer. Unpunctured vials may be held at room temperature for up to 24 hours. Use CDC’s beyond-use date labels for this vaccine to track storage time at refrigerated temperatures. Expiration Date To determine the expiration date, scan the QR code located on the vial or carton. The QR code will bring up a website; then choose the lookup option, enter the lot number, and the expiration date will be displayed. Another option is to access the website directly: http:// www.modernatx.com/covid19vaccine-eua. -

761111Orig1s000 PRODUCT QUALITY REVIEW(S)
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 761111Orig1s000 PRODUCT QUALITY REVIEW(S) Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research Office of Biotechnology Products First Approval for Indication/First Biosimilar/Expedited or Breakthrough Review: No Recommendation: Approval BLA Number: 761111 Review Number: 1 Review Date: April 16, 2020 Drug Name/Dosage Nyvepria- pegfilgrastim-apgf (PF-06881894); pre-filled syringe for single Form dose injection Strength/Potency 6 mg/0.6 mL (10 mg/1 mL) Route of Administration Subcutaneous injection Rx/OTC dispensed RX Indication All indications for US-licensed Neulasta Applicant/Sponsor Hospira Inc., a Pfizer Company US agent, if applicable n/a Product Overview: Nyvepria (PF-06881894; pegfilgrastim-apgf) is a covalent conjugate of recombinant methionyl human granulocyte-colony stimulating factor (G-CSF) (filgrastim) and a 20 kDa monomethoxypolyethylene glycol propionaldehyde (mPEG-p). PF-06881894 is a proposed biosimilar to the US-licensed Neulasta (pegfilgrastim). Endogenous G-CSF is the primary regulating factor for neutrophils. G-CSF binds to G-CSF receptors, which stimulates proliferation, differentiation, commitment, and target cell functional activation. Endogenous G-CSF is known to stimulate proliferation of mitotic cells, to reduce the maturation time of non-mitotic cells in the bone marrow, and to prolong the life span and enhance the function of mature neutrophils. Quality Review Team: Discipline Reviewer Branch/Division -

Influence of Sulfur for Oxygen Substitution in the Solvolytic Reactions of Chloroformate Esters and Related Compounds
Int. J. Mol. Sci. 2014, 15, 18310-18332; doi:10.3390/ijms151018310 OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Review Influence of Sulfur for Oxygen Substitution in the Solvolytic Reactions of Chloroformate Esters and Related Compounds Malcolm J. D’Souza 1,†,* and Dennis N. Kevill 2,†,* 1 Department of Chemistry, Wesley College, 120 N. State Street, Dover, DE 19901-3875, USA 2 Department of Chemistry and Biochemistry, Northern Illinois University, DeKalb, IL 60115-2862, USA † These authors contributed equally to this work. * Authors to whom correspondence should be addressed; E-Mails: [email protected] (M.J.D.); [email protected] (D.N.K.); Tel.: +1-302-736-2528 (M.J.D.); +1-815-753-6882 (D.N.K.); Fax: +1-302-736-2301 (M.J.D.); +1-815-753-4802 (D.N.K.). External Editor: Habil. Mihai V. Putz Received: 10 September 2014; in revised form: 22 September 2014 / Accepted: 29 September 2014 / Published: 10 October 2014 Abstract: The replacement of oxygen within a chloroformate ester (ROCOCl) by sulfur can lead to a chlorothioformate (RSCOCl), a chlorothionoformate (ROCSCl), or a chlorodithioformate (RSCSCl). Phenyl chloroformate (PhOCOCl) reacts over the full range of solvents usually included in Grunwald-Winstein equation studies of solvolysis by an addition-elimination (A-E) pathway. At the other extreme, phenyl chlorodithioformate (PhSCSCl) reacts across the range by an ionization pathway. The phenyl chlorothioformate (PhSCOCl) and phenyl chlorothionoformate (PhOCSCl) react at remarkably similar rates in a given solvent and there is a dichotomy of behavior with the A-E pathway favored in solvents such as ethanol-water and the ionization mechanism favored in aqueous solvents rich in fluoroalcohol. -

Photoremovable Protecting Groups in Chemistry and Biology: Reaction Mechanisms and Efficacy Petr Klan,́*,†,‡ Tomaś̌solomek,̌ †,‡ Christian G
Review pubs.acs.org/CR Photoremovable Protecting Groups in Chemistry and Biology: Reaction Mechanisms and Efficacy Petr Klan,́*,†,‡ Tomaś̌Solomek,̌ †,‡ Christian G. Bochet,§ Aurelień Blanc,∥ Richard Givens,⊥ Marina Rubina,⊥ Vladimir Popik,# Alexey Kostikov,# and Jakob Wirz∇ † Department of Chemistry, Faculty of Science, Masaryk University, Kamenice 5, 625 00 Brno, Czech Republic ‡ Research Centre for Toxic Compounds in the Environment, Faculty of Science, Masaryk University, Kamenice 3, 625 00 Brno, Czech Republic § Department of Chemistry, University of Fribourg, Chemin du Museé 9, CH-1700 Fribourg, Switzerland ∥ Institut de Chimie, University of Strasbourg, 4 rue Blaise Pascal, 67000 Strasbourg, France ⊥ Department of Chemistry, University of Kansas, 1251 Wescoe Hall Drive, 5010 Malott Hall, Lawrence, Kansas 66045, United States # Department of Chemistry, University of Georgia, Athens, Georgia 30602, United States ∇ Department of Chemistry, University of Basel, Klingelbergstrasse 80, CH-4056 Basel, Switzerland 7.3. Arylsulfonyl Group 160 7.4. Ketones: 1,5- and 1,6-Hydrogen Abstraction 160 7.5. Carbanion-Mediated Groups 160 7.6. Sisyl and Other Silicon-Based Groups 161 7.7. 2-Hydroxycinnamyl Groups 161 7.8. α-Keto Amides, α,β-Unsaturated Anilides, CONTENTS and Methyl(phenyl)thiocarbamic Acid 162 7.9. Thiochromone S,S-Dioxide 162 1. Introduction 119 7.10. 2-Pyrrolidino-1,4-Benzoquinone Group 162 2. Arylcarbonylmethyl Groups 121 7.11. Triazine and Arylmethyleneimino Groups 162 2.1. Phenacyl and Other Related Arylcarbonyl- 7.12. Xanthene and Pyronin Groups 163 methyl Groups 121 o 7.13. Retro-Cycloaddition Reactions 163 2.2. -Alkylphenacyl Groups 123 8. Sensitized Release 163 2.3. p-Hydroxyphenacyl Groups 125 p 8.1. -

Non-Covalent Pegylation of L-Asparaginase Using Pegylated
Page 1 of 25 Journal of Pharmaceutical Sciences 1 Non-covalent PEGylation of L-asparaginase using 2 PEGylated polyelectrolyte 3 4 Takaaki Kurinomaru and Kentaro Shiraki * 5 Faculty of Pure and Applied Sciences, University of Tsukuba, 1-1-1 Tennodai, Tsukuba, Ibaraki 6 305-8573, Japan 7 8 To whom correspondence should be addressed. Tel.: +81-29-8535306. Fax: +81-29-8535215. E- 9 mail: [email protected] 10 1 Journal of Pharmaceutical Sciences Page 2 of 25 11 Abstract 12 Non-covalent PEGylation has great potential for stabilization of therapeutic proteins. Here, 13 we demonstrated that the non-covalent PEGylation with a PEGylated polyelectrolyte stabilized a 14 therapeutic protein, L-asparaginase (ASNase). Anionic ASNase and cationic poly(ethylene glycol)- 15 block -poly( N,N -dimethylaminoethyl methacrylate) (PEG-b-PAMA) formed a water-soluble protein– 16 polyelectrolyte complex (PPC) without loss of secondary structure and enzyme activity. PPC with 17 PEG-b-PAMA successfully inhibited the shaking-induced inactivation and aggregation of ASNase 18 as well as protease digestion, corresponding to the behaviors of covalently PEGylated ASNase. Thus, 19 non-covalent PEGylation by PEGylated polyelectrolytes is a new candidate for handling of 20 therapeutic proteins. 21 22 23 Keywords 24 Complexation, Pegylation, Polyelectrolytes, Protein aggregation, Protein formulation, Stabilization 25 26 Abbreviations 27 L-Asn, L-asparagine; ASNase, L-asparaginase; CD, circular dichroism; DLS, dynamic light 28 scattering; MOPS, 3-(N-morpholino)propanesulfonic -

Studies on Polyethylene Glycol-Monoclonal Antibody Conjugates for Fabrication of Nanoparticles for Biomedical Applications
RESEARCH ARTICLE Studies on polyethylene glycol-monoclonal antibody conjugates for fabrication of nanoparticles for biomedical applications Funmilola Fisusi, Nailah Brandy, Jingbo Wu, Emmanuel O Akala Fisusi F, Brandy N, Jingbo Wu and Akala EO. Studies on polyethylene that there are two PEGs per each antibody and it appears more reliable than glycol-monoclonal antibody conjugates for fabrication of nanoparticles for the degree of SAMSA-fluorescein substitution method. HER-2 binding assay biomedical applications. J Nanosci Nanomed January 2020;4(2):1-9. showed that PEGylated monoclonal antibody bound less efficiently to SKBR3 (high HER-2 expressing) cells than unmodified trastuzumab and OBJECTIVE: The objective of this work is to synthesize and characterize pertuzumab. In vitro growth inhibitory effects of unmodified monoclonal PEGylated monoclonal antibody using the reactivity of oligosaccharide antibodies increased with increase in concentration; while the in vitro residues in the Fc region of trastuzumab and pertuzumab with a view to growth inhibitory effects of PEGylated monoclonal antibodies also preserving their activities. increased (but less than the pure antibody) with concentration and it METHODS: The hydrazide-functionalized PEG monomethacrylate was appeared to be more active than unmodified mAbs at higher concentration. synthesized and reacted with NaIO4-generated aldehyde groups on glycans in CONCLUSION: The results indicate that PEG can be site-specifically the Fc-domain of trastuzumab and pertuzumab. The conjugates were attached via the oxidized glycans in the Fc domain of monoclonal purified by HPLC. SAMSA-fluorescein substitution method and MALDI antibodies but the process needs further optimization in terms of PEG size MS spectroscopy were used to determine the number of PEG per antibody. -
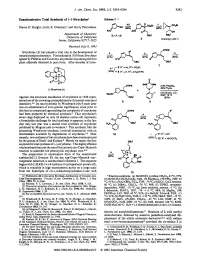
Enantioselective Total Synthesis of (-)-Strychnine1 Scheme1
J. Am. Chem. SOC.1993,115, 9293-9294 9293 Enantioselective Total Synthesis of (-)-Strychnine1 Scheme1 Steven D. Knight, Larry E. Overman,' and Garry Pairaudeau Department of Chemistry University of California Imine, California 9271 7-2025 Received July 8, 1993 OTlPS Strychnine (1) has played a vital role in the development of d h,i natural productschemistry. First isolated in 1818 fromstrychnos ignatii by Pelletier and Caventou, strychnine was among the first plant alkaloids obtained in pure form. After decades of inves- OBU' (-)-Strychnine(1) tigation, the structural elucidation of strychnine in 1946 repre- sented one of the crowning accomplishments of classical structural chemistry.394 Its total synthesis by Woodward only 8 years later was an achievement of even greater significance, since prior to this feat no compound approaching the complexity of strychnine ,oms had been prepared by chemical synthesis.* That strychnine's P' seven rings displayed on only 24 skeletal atoms still represents a formidable challenge for total synthesis is apparent in the fact that only last year was a second total synthesis of strychnine published by Magnus and co-workers.6 This synthesis, like the pioneering Woodward synthesis, involved intersection with an intermediate available by degradation of stry~hnine.~.~Most recently, two syntheses of (*)-strychnine have been communicated by the groups of Stork' and Kuehne.* Herein we report the first asymmetric total synthesis of (-)-strychnine. This highly efficient total synthesis features the use of the cationic aza-Cope-Mannich reaction to assemble the pentacyclic strychnan c~re.~J~ The preparation in enantiopure form of the unsaturated azabicyclo[3.2.