Medical Management of HIV Infection Bartlett.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Safety and Efficacy Trial of Circumferential Anal Canal Radiofrequency Ablation for High-Grade Anal Intraepithelial Neoplasia
Protocol 01-2017: RFA for Anal Intraepithelial Neoplasia A Safety and Efficacy Trial of Circumferential Anal Canal Radiofrequency Ablation for High-Grade Anal Intraepithelial Neoplasia Using The BARRX™ Anorectal Wand (Clinical Protocol 01-2017) AUGUST 31, 2017 Page 1 of 40 Protocol 01-2017: RFA for Anal Intraepithelial Neoplasia PROTOCOL SIGNATURE PAGE I, , Principal Investigator at site , agree to conduct and follow this protocol: PROTOCOL 01-2017: A Safety and Efficacy Trial of Circumferential Anal Canal Radiofrequency Ablation for Anal Intraepithelial Neoplasia using the Barrx™ Anorectal Wand as written according to FDA guidelines. I understand that no deviations from the above protocol may be made without written permission from the Protocol Chair(s). _________________________________ _____________________ Signature Date (mm/dd/yyyy) Page 2 of 40 Protocol 01-2017: RFA for Anal Intraepithelial Neoplasia P ROT O COL S U M M ARY Title A Safety and Efficacy Trial of Circumferential Anal Canal Radiofrequency Ablation for Anal Intraepithelial Neoplasia using the Barrx™ Anorectal Wand Design Multi-center prospective trial involving up to 70 subjects Subject Population HIV-positive and HIV-negative subjects with intra-anal intraepithelial neoplasia (AIN) containing at least one high- grade squamous intraepithelial lesions (HSIL) involving the squamocolumnar junction (SCJ). Objective Assess the safety, and efficacy of circumferential radiofrequency ablation (RFA) to the anal canal using the FDA cleared Barrx™ Anorectal Wand to eradicate anal -
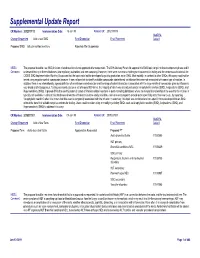
Detail Report
Supplemental Update Report CR Number: 2012319113 Implementation Date: 16-Jan-19 Related CR: 2012319113 MedDRA Change Requested Add a new SMQ Final Disposition Final Placement Code # Proposed SMQ Infusion related reactions Rejected After Suspension MSSO The proposal to add a new SMQ Infusion related reactions is not approved after suspension. The ICH Advisory Panel did approve this SMQ topic to go into the development phase and it Comment: underwent testing in three databases (two regulatory authorities and one company). However, there were numerous challenges encountered in testing and the consensus decision of the CIOMS SMQ Implementation Working Group was that the topic could not be developed to go into production as an SMQ. Most notably, in contrast to other SMQs, this query could not be tested using negative control compounds because it was not possible to identify suitable compounds administered via infusion that were not associated with some type of reaction. In addition, there is no internationally agreed definition of an infusion related reaction and the range of potential reactions associated with the large variety of compounds given by infusion is very broad and heterogenous. Testing was conducted on a set of around 500 terms, the majority of which was already included in Anaphylactic reaction (SMQ), Angioedema (SMQ), and Hypersensitivity (SMQ). It proved difficult to identify potential cases of infusion related reactions in post-marketing databases where the temporal relationship of the event to the infusion is typically not available. In clinical trial databases where this information is more easily available, users are encouraged to provide more specificity about the event, e.g., by reporting “Anaphylactic reaction” when it is known that this event is temporally associated with the infusion. -

Download Article PDF/Slides
Kan Lu, PharmD New Antiretrovirals for Based on a presentation at prn by Roy M. Gulick, md, mph the Treatment of HIV: Kan Lu, PharmD | Drug Development Fellow University of North Carolina School of Pharmacy Chapel Hill, North Carolina The View in 2006 Roy M. Gulick, md, mph Reprinted from The prn Notebook® | october 2006 | Dr. James F. Braun, Editor-in-Chief Director, Cornell Clinical Trials Unit | Associate Professor of Medicine, Meri D. Pozo, PhD, Managing Editor. Published in New York City by the Physicians’ Research Network, Inc.® Weill Medical College of Cornell University | New York, New York John Graham Brown, Executive Director. For further information and other articles available online, visit http://www.prn.org | All rights reserved. ©october 2006 substantial progress continues to be made in the arena of cokinetics and a long extracellular half-life of approximately 10 hours antiretroviral drug development. prn is again proud to present its annual (Zhu, 2003). During apricitabine’s development, a serious drug interac- review of the experimental agents to watch for in the coming months and tion with lamivudine (Epivir) was noted. Although the plasma years. This year’s review is based on a lecture by Dr. Roy M. Gulick, a long- concentrations of apricitabine were unaffected by coadministration of time friend of prn, and no stranger to the antiretroviral development lamivudine, the intracellular concentrations of apricitabine were reduced pipeline. by approximately sixfold. Additionally, the 50% inhibitory concentration To date, twenty-two antiretrovirals have been approved by the Food (ic50) of apricitabine against hiv with the M184V mutation was increased and Drug Administration (fda) for the treatment of hiv infection. -

Ep 2531027 B1
(19) TZZ ¥_Z _T (11) EP 2 531 027 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 31/4985 (2006.01) A61K 31/52 (2006.01) 06.05.2015 Bulletin 2015/19 A61K 31/536 (2006.01) A61K 31/513 (2006.01) A61K 38/55 (2006.01) A61P 31/18 (2006.01) (21) Application number: 11737484.3 (86) International application number: (22) Date of filing: 24.01.2011 PCT/US2011/022219 (87) International publication number: WO 2011/094150 (04.08.2011 Gazette 2011/31) (54) Therapeutic combination comprising dolutegravir, abacavir and lamivudine Therapeutische Zusammensetzung enthaltend Dolutegravir, Abacavir und Lamivudine Combinaison thérapeutique comprenant du dolutégravir, de l’abacavir et de la lamivudine (84) Designated Contracting States: (74) Representative: Gladwin, Amanda Rachel AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GlaxoSmithKline GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO Global Patents (CN925.1) PL PT RO RS SE SI SK SM TR 980 Great West Road Designated Extension States: Brentford, Middlesex TW8 9GS (GB) BA ME (56) References cited: (30) Priority: 27.01.2010 US 298589 P WO-A1-2010/011812 WO-A2-2009/148600 US-A1- 2006 084 627 US-A1- 2006 084 627 (43) Date of publication of application: US-A1- 2008 076 738 US-A1- 2009 318 421 12.12.2012 Bulletin 2012/50 US-A1- 2009 318 421 US-B1- 6 544 961 (73) Proprietor: VIIV Healthcare Company • SONG1 et al: "The Effect of Ritonavir-Boosted Research Triangle Park, NC 27709 (US) ProteaseInhibitors on the HIV Integrase Inhibitor, S/GSK1349572,in Healthy Subjects", INTERNET , (72) Inventor: UNDERWOOD, Mark, Richard 15 September 2009 (2009-09-15), XP002697436, Research Triangle Park Retrieved from the Internet: URL:http: North Carolina 27709 (US) //www.natap.org/2009/ICCAC/ICCAC_ 52.htm [retrieved on 2013-05-21] Note: Within nine months of the publication of the mention of the grant of the European patent in the European Patent Bulletin, any person may give notice to the European Patent Office of opposition to that patent, in accordance with the Implementing Regulations. -

Frequency and Implications of HIV Superinfection
Review Frequency and implications of HIV superinfection Andrew D Redd, Thomas C Quinn, Aaron A R Tobian* HIV superinfection occurs when an individual with HIV is infected with a new distinct HIV viral strain. Superinfection Published Online has been reported throughout the world, and studies have recorded incidence rates of 0–7∙7% per year. Use of next- May 31, 2013 generation sequencing has improved detection of superinfection, which can be transmitted by injecting drug use and http://dx.doi.org/10.1016/ S1473-3099(13)70066-5 sexual intercourse. Superinfection might have incidence rates comparable to those of initial HIV infection. Clinicians Division of Intramural should encourage safe sexual and injecting drug use practices for HIV-infected patients because superinfection has Research, National Institute of detrimental eff ects on clinical outcomes and could pose a concern for large-scale antiretroviral treatment plans. The Allergy and Infectious Diseases, occurrence of superinfection has implications for vaccine research, since it seems initial HIV infection is not fully National Institutes of Health, protective against a subsequent infection. Additional collaborative research could benefi t care of patients and inform Bethesda, MD, USA (A D Redd PhD, future vaccine design. Prof T C Quinn MD); Department of Pathology, Introduction Detection of HIV superinfection School of Medicine, Johns HIV superinfection occurs when an individual with The investigators in initial studies that identifi ed indi- Hopkins University, Baltimore, MD (A A R Tobian MD) HIV becomes infected with a new, phylogenetically distinct viduals dually infected with HIV-1 and HIV-2 used Correspondence to: viral HIV strain. -

Microbicides 2008
State of the Network Ian McGowan & Sharon Hillier Annual Meeting March 14th, 2016 HIV Infection in the US HIV Infection in US MSM HIV Infection in US Women Global HIV Infection HIV Incidence Rates in ASPIRE & RING Studies ASPIRE Study RING Study 10 8 6 4 2 HIV Incidence Rates (%) Rates Incidence HIV 0 <21 years > 25 years 18-21 years22-26 years27-45 years 21-25 years The PrEP Landscape in 2016 (1) • Oral PrEP – Optimization of Truvada delivery – Increasing availability – US, Canada, France, South Africa, Kenya, Israel – 2nd generation regimens being evaluated • Maraviroc combinations • Tenofovir alafenamide – New molecules • EFdA (Merck) The PrEP Landscape in 2016 (2) • Injectable PrEP – Rilpivirine & Cabotegravir • Intravaginal rings – Phase 3 • Dapivirine – Phase 1/2 • Tenofovir • Tenofovir disoproxil fumarate • Vicriviroc + MK 2048 • Dapivirine + Levonorgestrol The PrEP Landscape in 2016 (3) • Rectal microbicides – Phase 2 • Reduced glycerin tenofovir gel – Phase 1 • Maraviroc • Dapivirine • Griffithsin • MIV-150 / Carageenan / Zinc • Implantable PrEP MTN Highlights from the Past Year The MTN Portfolio 2015 / 2016 Studies in Ongoing development: Completed studies MTN-026 studies MTN-030 MTN-015 MTN-031 MTN-017 MTN-016 MTN-032 MTN-020 MTN-023 MTN-033 MTN-024 MTN-027 MTN-028 MTN-034 MTN-029 MTN-035 MTN-036 MTN-037 Intravaginal Ring Studies • Dapivirine • Vicriviroc / MK-2048 – MTN-020 – MTN-027 & MTN-028 – MTN-023 • Dapivirine 200 mg – MTN-025 (HOPE) IVR – MTN-029 – MTN-036 / IPM 047 – MTN-031 – MTN-034 • Dapivirine / Levonorgestrel – MTN-030 / IPM 041 Rectal Microbicide Studies • Tenofovir gel – MTN-017 • Dapivirine gel – MTN-026 – MTN-033 – MTN-035 CHARM-02 Study (Hiruy H et al. -

Semen-Mediated Enhancement of HIV-1 Infection Markedly Impairs the Antiviral Efficacy of Microbicides
Institute of Molecular Virology Ulm University Medical Center Prof. Dr. Frank Kirchhoff Semen-mediated enhancement of HIV-1 infection markedly impairs the antiviral efficacy of microbicides Dissertation to obtain the Doctoral Degree of Human Biology (Dr. biol. hum.) at the Faculty of Medicine, University of Ulm presented by Onofrio Zirafi Heidenheim an der Brenz 2014 Present Dean: Prof. Dr. Thomas Wirth 1st reviewer: Prof. Dr. Jan Münch 2nd reviewer: Prof. Dr. Barbara Spellerberg Date of graduation: 5th December 2014 TABLE OF CONTENTS III TABLE OF CONTENTS ABBREVIATIONS ............................................................................................................. V 1 INTRODUCTION ....................................................................................................... 1 1.1 HIV LIFE CYCLE AND ANTIRETROVIRAL DRUGS .......................................................... 2 1.2 HIV TRANSMISSION ................................................................................................... 4 1.3 FACTORS IN SEMEN MODULATING HIV-1 INFECTION .................................................. 5 1.4 MECHANISM OF INFECTION ENHANCEMENT BY SEMINAL AMYLOIDS ........................... 6 1.5 COUNTERACTING AMYLOID-MEDIATED ENHANCEMENT OF HIV-1 INFECTION ............ 6 1.6 STRUCTURE-BASED PEPTIDE INHIBITORS OF AMYLOID FORMATION ............................. 7 1.7 MICROBICIDES ............................................................................................................ 8 1.8 SCIENTIFIC AIM ........................................................................................................ -

Coevolution of HIV-1 and Broadly Neutralizing Antibodies
HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Curr Opin Manuscript Author HIV AIDS. Author Manuscript Author manuscript; available in PMC 2020 October 13. Published in final edited form as: Curr Opin HIV AIDS. 2019 July ; 14(4): 286–293. doi:10.1097/COH.0000000000000550. Coevolution of HIV-1 and broadly neutralizing antibodies Nicole A. Doria-Rosea, Elise Landaisb aVaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland bIAVI Neutralizing Antibody Center, Immunology and Microbiology Department, The Scripps Research Institute, La Jolla, California, USA Abstract Purpose of review—Exploring the molecular details of the coevolution of HIV-1 Envelope with broadly neutralizing antibodies (bNAbs) in infected individuals over time provides insights for vaccine design. Since mid-2017, the number of individuals described in such publications has nearly tripled. New publications have extended such studies to new epitopes on Env and provided more detail on previously known sites. Recent findings—Studies of two donors – one of them an infant, the other with three lineages targeting the same site – has deepened our understanding of V3-glycan-directed lineages. A V2- apex-directed lineage showed remarkable similarity to a lineage from a previously described donor, revealing general principles for this class of bNAbs. Understanding development of CD4 binding site antibodies has been enriched by the study of a VRC01-class lineage. Finally, the membrane-proximal external region is a new addition to the set of epitopes studied in this manner, with early development events explored in a study of three lineages from a single donor. -

Infectious Gastroenteritis Generalised Anxiety Disorder HIV Smoking Associated Cancers
BEST PRACTICE 25 DECEMBER 2009 Infectious gastroenteritis Generalised anxiety disorder HIV bpac nz Smoking associated cancers better medicin e Editorial Team Tony Fraser Professor Murray Tilyard We would like to acknowledge the following people for Clinical Advisory Group their guidance and expertise in developing this edition: Michele Cray Dr Shaun Costello, Dunedin Serena Curtis-Lemuelu Dr Edward Coughlan, Christchurch Dr Rosemary Ikram Professor Tony Dowell, Wellington Dr Cam Kyle Dr Rosemary Ikram, Christchurch Dr Chris Leathart Mr William Pearce, Christchurch Dr Lynn McBain Dr Alan Pithie, Christchurch Adam McRae Dr Gabrielle Ruben, Wellington Janet Maloney-Moni Assoc. Professor Mark Thomas, Auckland Dr Peter Moodie Dr Robyn Toomath, Wellington Associate Professor Jim Reid Dr Neil Whittaker, GP Reviewer, Nelson Associate Professor David Reith Assoc. Professor Michael Williams, Dunedin Professor Murray Tilyard Programme Development Team Rachael Clarke Peter Ellison Best Practice Journal (BPJ) Rebecca Harris Julie Knight ISSN 1177-5645 Noni Richards BPJ, Issue 25, December 2009 Dr Tom Swire Dr AnneMarie Tangney nz Dr Sharyn Willis BPJ is published and owned by bpac Ltd Dave Woods Level 8, 10 George Street, Dunedin, New Zealand. Report Development Team Bpacnz Ltd is an independent organisation that promotes health Justine Broadley care interventions which meet patients’ needs and are evidence Todd Gillies based, cost effective and suitable for the New Zealand context. Lana Johnson We develop and distribute evidence based resources which describe, facilitate and help overcome the barriers to best Web practice. Gordon Smith Bpacnz Ltd is currently funded through contracts with PHARMAC Design and DHBNZ. Michael Crawford Bpacnz Ltd has five shareholders: Procare Health, South Link Management and Administration Health, IPAC, the University of Otago and Pegasus Health. -

Lessons from Viral Superinfections for HIV-1 Vaccine Design Stephanie Jost* Ragon Institute of MGH, MIT and Harvard, USA
C S & lini ID ca A l f R o e l s Journal of a e n a r r Jost, J AIDS Clinic Res 2013, S3 c u h o J DOI: 10.4172/2155-6113.S3-005 ISSN: 2155-6113 AIDS & Clinical Research Review Article Open Access Lessons from Viral Superinfections for HIV-1 Vaccine Design Stephanie Jost* Ragon Institute of MGH, MIT and Harvard, USA Abstract Superinfection refers to a second viral infection in the context of a pre-existing adaptive immune response to prior infection with a viral strain that has not been cleared, the two viruses being genetically distinct yet belonging to the same genus. As such, this phenomenon provides unique settings to gain insights into the immune correlates of protection against HIV-1. The focus of this review is to discuss the current knowledge about immune responses to HIV-1 and to other viruses that are associated with partial or complete immunity to superinfection, or lack thereof, and how that could be applied to future HIV-1 vaccine strategies. Keywords: HIV-1; Superinfection; Vaccine; Co-infection; Viral HIV-1 Rapid Evolution and Diversity: A Challenge for infection; Recombination Vaccine Design Introduction The extremely rapid evolution of the virus probably largely contributed to the failure or limited success of HIV-1 vaccines evaluated The human immunodeficiency virus type 1 (HIV-1) affects 34 to date [5]. HIV-1’s extensive genetic diversity was first brought to light million adults and children worldwide, and the ongoing spread of the around 1983, when full-length sequences of the virus became available, epidemic resulted in about 2.5 million new infections and 1.7 million and has since then expanded [6]. -

Pathology of Anal Cancer
Pathology of Anal Cancer a b Paulo M. Hoff, MD, PhD , Renata Coudry, MD, PhD , a, Camila Motta Venchiarutti Moniz, MD * KEYWORDS Anal cancer Anal squamous intraepithelial neoplasia Squamous cell carcinoma Human papilloma virus (HPV) Molecular KEY POINTS Anal cancer is an uncommon tumor, squamous cell carcinoma (SCC) being the most frequent histology corresponding to 80% of all cases. Human papilloma virus (HPV) infection plays a key role in anal cancer development, en- coding at least three oncoproteins with stimulatory properties. SCC expresses CK5/6, CK 13/19, and p63. P16 is a surrogate marker for the presence of HPV genome in tumor cells. INTRODUCTION Anal cancer accounts for approximately 2.4% of gastrointestinal malignancies.1 Although anal cancer is a rare tumor, its frequency is increasing, especially in high- risk groups.2 Tumors in this location are generally classified as anal canal or anal margin. Squamous cell carcinoma (SCC) is the predominant type of tumor and shares many features with cervical cancer. Oncogenic human papilloma virus (HPV) infection plays a major role in both tumors.3 HIV infection is associated with a higher frequency of HPV-associated premalignant lesions and invasive tumors.4 Normal Anatomy of the Anus The anal canal is the terminal part of the large intestine and is slightly longer in male than in female patients. It measures approximately 4 cm and extends from the rectal ampulla (pelvic floor level) to the anal verge, which is defined as the outer open- ing of the gastrointestinal tract. The anal verge is at the level of the squamous- mucocutaneous junction with the perianal skin.5,6 The authors have nothing to disclose. -

HPV, Anal Dysplasia and Anal Cancer
HPV, anal dysplasia FACT and anal cancer SHEET Published 2016 Summary Anal cancer typically develops over a period of years, beginning with a precancerous condition called anal dysplasia. CONTACT US Anal dysplasia occurs when clusters of abnormal cells form by telephone lesions in the mucosa lining of the anal canal (between the 1-800-263-1638 anus and the rectum). The lesions typically form inside the anal 416-203-7122 canal or just outside the anal opening. by fax 416-203-8284 Although there are over 100 different types of the human papillomavirus (HPV), anal dysplasia is usually caused by certain by e-mail strains of HPV which can be transmitted sexually. HPV can shut [email protected] off the proteins that help prevent dysplasia and cancer cells from developing, therefore leading to HPV-associated diseases by mail such as anal dysplasia. 555 Richmond Street West Suite 505, Box 1104 It is difficult to screen for anal dysplasia since the lesions are Toronto ON M5V 3B1 not detectable by routine examinations. As a result, anal dysplasia is often not detected until it has developed into anal cancer, which can be difficult to treat depending on the severity. Specific screening tests can detect dysplasia or precancerous changes. If these precancers are treated, anal cancer may be prevented. Anal cancer is usually treated with radiation and chemotherapy or with surgery. Although anal dysplasia may be treated successfully, individuals with HIV are at increased risk of it recurring and may need to be monitored closely by a trained physician. Consistent condom use reduces, but does not eliminate, the risk of transmitting HPV.