University of Florida Thesis Or Dissertation Formatting
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Accused Persons Arrested in Thrissur City District from 12.06.2016 to 18.06.2016
Accused Persons arrested in Thrissur City district from 12.06.2016 to 18.06.2016 Name of Name of the Name of the Place at Date & Arresting Court at Sl. Name of the Age & Cr. No & Sec Police father of Address of Accused which Time of Officer, which No. Accused Sex of Law Station Accused Arrested Arrest Rank & accused Designation produced 1 2 3 4 5 6 7 8 9 10 11 KOLAPPULLY HOUSE, 2078/16 U/S TOWN EAST 12.06.2016 M K AJAYAN, SI BAILED BY 1 RAGESH K R RAJAN 29 MALE MULAYAM P O, DIVANJIMOOLA 15(C) R/W 63 PS (THRISSUR at 00.15 OF POLICE POLICE VALAKKAVU ABKARAI ACT CITY) AMBATT HOUSE, 2079/16 U/S TOWN EAST 12.06.2016 M K AJAYAN, SI BAILED BY 2 VARGHESE A T THOMAS 46 MALE MULAYAM P O , DIVANJIMOOLA 15(C) R/W 63 PS (THRISSUR at 00.22 OF POLICE POLICE VALAKKAVU ABKARAI ACT CITY) MELAYIL HOUSE, 2080/16 U/S TOWN EAST RAMACHAND 12.06.2016 M K AJAYAN, SI BAILED BY 3 RAMAN 47 MALE MULAYAM P O , DIVANJIMOOLA 15(C) R/W 63 PS (THRISSUR RAN at 00.30 OF POLICE POLICE VALAKKAVU ABKARAI ACT CITY) MULLOOKKARAN 2081/16 U/S T OWN EAST 12.06.2016 M.K. AJAYAN, BAILED BY 4 SHIJI RAPPAI 39 MALE HOUSE, MULAYAM DIVANJIMOOLA 15(C) R/W 63 PS (THRISSUR AT 00.29 SI OF POLICE POLICE VALAKKAVU ABKARAI ACT CITY) PALUKKASSERY 2082/16 U/S TOWN EAST CHANDRASEKH 12.06.2016 M K AJAYAN, SI BAILED BY 5 RAJKUMAR 48 MALE HOUSE, MULAYAM P DIVANJIMOOLA 15(C) R/W 63 PS (THRISSUR ARAN at 00.50 OF POLICE POLICE O , VALAKKAVU ABKARAI ACT CITY) THACHATTIL HOUSE,NEAR 2084/16 U/S TOWN EAST V.K. -

State District Branch Address Centre Ifsc Contact1 Contact2 Contact3 Micr Code Andhra Pradesh East Godavari Rajamundry Pb No
STATE DISTRICT BRANCH ADDRESS CENTRE IFSC CONTACT1 CONTACT2 CONTACT3 MICR_CODE M RAGHAVA RAO E- MAIL : PAUL RAJAMUN KAKKASSERY PB NO 23, FIRST DRY@CSB E-MAIL : FLOOR, STADIUM .CO.IN, RAJAMUNDRY ROAD, TELEPHO @CSB.CO.IN, ANDHRA EAST RAJAMUNDRY, EAST RAJAHMUN NE : 0883 TELEPHONE : PRADESH GODAVARI RAJAMUNDRY GODAVARY - 533101 DRY CSBK0000221 2421284 0883 2433516 JOB MATHEW, SENIOR MANAGER, VENKATAMATTUPAL 0863- LI MANSION,DOOR 225819, NO:6-19-79,5&6TH 222960(DI LANE,MAIN R) , CHANDRAMOH 0863- ANDHRA RD,ARUNDELPET,52 GUNTUR@ ANAN , ASST. 2225819, PRADESH GUNTUR GUNTUR 2002 GUNTUR CSBK0000207 CSB.CO.IN MANAGER 2222960 D/NO 5-9-241-244, Branch FIRST FLOOR, OPP. Manager GRAMMER SCHOOL, 040- ABID ROAD, 23203112 e- HYDERABAD - mail: ANDHRA 500001, ANDHRA HYDERABA hyderabad PRADESH HYDERABAD HYDERABAD PRADESH D CSBK0000201 @csb.co.in EMAIL- SECUNDE 1ST RABAD@C FLOOR,DIAMOND SECUNDER SB.CO.IN TOWERS, S D ROAD, ABAD PHONE NO ANDHRA SECUNDERABA DECUNDERABAD- CANTONME 27817576,2 PRADESH HYDERABAD D 500003 NT CSBK0000276 7849783 THOMAS THARAYIL, USHA ESTATES, E-MAIL : DOOR NO 27.13.28, VIJAYAWA NAGABHUSAN GOPALAREDDY DA@CSB. E-MAIL : ROAD, CO.IN, VIJAYAWADA@ GOVERNPOST, TELEPHO CSB.CO.IN, ANDHRA VIJAYAWADA - VIJAYAWAD NE : 0866 TELEPHONE : PRADESH KRISHNA VIJAYAWADA 520002 A CSBK0000206 2577578 0866 2571375 MANAGER, E-MAIL: NELLORE ASST.MANAGE @CSB.CO. R, E-MAIL: PB NO 3, IN, NELLORE@CS SUBEDARPET ROAD, TELEPHO B.CO.IN, ANDHRA NELLORE - 524001, NE:0861 TELEPHONE: PRADESH NELLORE NELLORE ANDHRA PRADESH NELLORE CSBK0000210 2324636 0861 2324636 BR.MANAG ER : PHONE :040- ASST.MANAGE 23162666 R : PHONE :040- 5-222 VIVEKANANDA EMAIL 23162666 NAGAR COLONY :KUKATPA EMAIL ANDHRA KUKATPALLY KUKATPALL LLY@CSB. -

Of Thrissur City Sl
Weekly Consolidated list of arrested persons (22.02.2015 to 28.02.2015) of Thrissur City Sl. Name of the Name of the Father of Age & Address of accused Place at which Date & Time of Crim No. & Sec of Police Station Name of Arresting Name of the No Accused Accused Sex arrested Arrest Law Officer Court at which accused produced 1 Ranjith Raghavan 33 Male Arakkal House, Athekkad Athekkad 23.02.2015 Cr. 246/15 U/s 151 Viyyur K. K. Mohandas, SI of JFCM I, Thrissur Desom, Kolazhy Village at 09.00 Hrs CrPC Police, Viyyur 2 Sanesh Chandran 31 Male Chettaltamveetil House, Ayyanthole 24-02-2015 Cr. 245/15 U/s 408, Thrissur West P. R. Bejoy, SI of CJM Court, Chombala P.o, Vadakkara at 14.30 Hrs 418, 420 IPC Police, Thrissur West Thrissur Kozhikode 3 Lijo Poulouse 28 Male Thundiyil Veedu, Thamburattimoola 24.02.2015 Cr. 45/15 u/s 461, 457, Ollur M. G. Ponnappan, SI JFCM III, Thrissur Thamburattimoola, Puthur at 08.00 Hrs 380 IPC of Police, Ollur 4 Ranjith Unnimon 23 Male Elembalamkattil House, Peramangalam 24-02-2015 Cr. 280/15 U/s 366(a), Peramangalam T. N. Sudhakaran, SI JFCM, Kuzhikkattukonnam, at 10.00 Hrs 376 IPC & 3, 4, 5 of of Police, Kunnamkulam Madayikonnam POCSO Act-2012 Peramangalam 5 Ratheesh Sudhakaran 33 Male Kadavarath House, Peramangalam 24-02-2015 Cr. 292/15 U/s 447, Peramangalam T. N. Sudhakaran, SI JFCM, Ambedkar Nagar, Avanur at 09.00 hrs 326, 506(ii) IPC of Police, Kunnamkulam Peramangalam 6 Aneesh Anandan 28 Male Aranattil (H) Aloor (V) Guruvayur 25.02.2015 Cr.202/15 U/s 341 323 Guruvayur M Sasidharan, SI of JFCM, Chavakkad -

Physicochemical Characteristics of Soils of Talapilli Taluk, Thrissur
Saudi Journal of Humanities and Social Sciences ISSN 2415-6256 (Print) Scholars Middle East Publishers ISSN 2415-6248 (Online) Dubai, United Arab Emirates Website: http://scholarsmepub.com/ Physicochemical Characteristics of Soils of Talapilli Taluk, Thrissur, Kerala, India Shyju K1, Kumaraswamy K2 1UGC BSR Research Scholar & 2UGC BSR Faculty Fellow Department of Geography, Bharathidasan University, Tiruchirappalli, Tamil Nadu, India Abstract: The importance of soil resources to attain sustainability in crop *Corresponding author production, eco-development and protection of environment is an established fact. Shyju K Management of soil on a scientific basis is essential for sustained and increased agricultural production, on this prime perspective a study on physiochemical Article History characters of soils has been conducted in the Talapilli Taluk of Thrissur District, Received: 22.11.2017 India. Talapilli is the agricultural dominant area in the District and hence the study Accepted: 27.11.2017 help in more understanding of the quality of the soil for proper management Published: 30.11.2017 practices in agriculture and other sectors as well. The physiochemical characters analyzed in the study are texture, soil depth, slope, erosion, pH, electrical DOI: conductivity, primary nutrients like available nitrogen, phosphorus and potassium 10.21276/sjhss.2017.2.11.15 and secondary nutrients like magnesium and sulphur. The result indicates that Talapilli has gravelly clay loam and gravelly sandy clay loam soils in higher proportion having moderate to deep soil depth with moderate slope and less to moderate intensity of erosion. As far as chemical composition is concerned in Talapilli Taluk major proportion of soil has pH ranges from 5.1 to 6, that which shows the acidic nature of the soil. -

Accused Persons Arrested in Thrissur City District from 21.06.2020To27.06.2020
Accused Persons arrested in Thrissur City district from 21.06.2020to27.06.2020 Name of Name of the Name of the Place at Date & Arresting Court at Sl. Name of the Age & Cr. No & Sec Police father of Address of Accused which Time of Officer, which No. Accused Sex of Law Station Accused Arrested Arrest Rank & accused Designation produced 1 2 3 4 5 6 7 8 9 10 11 3091/2020 U/s 269, 290 IPC & 118(e) of KP Act & Thrissur Parambhil 27-06-2020 Sec. 4(2)(d) 29, East BAILED BY 1 Sovin Sojan tharakkan house, Jose junction at 23:10 r/w 5 of Bibin c v Male (Thrissur POLICE ollur desam, ollur Hrs Kerala City) Epidemic Diseases Ordinance 2020 3090/2020 U/s 269, 290 IPC & 118(e) of KP Act & Thrissur 27-06-2020 Sec. 4(2)(d) 18, Panikkavettil house, East BAILED BY 2 Asab Latheef Jose junction at 22:50 r/w 5 of Bibin c v Male kalathode (Thrissur POLICE Hrs Kerala City) Epidemic Diseases Ordinance 2020 3089/2020 U/s 269, 290 IPC & 118(e) of KP Act & VIMALALAYAM Thrissur NIRMAL 27-06-2020 Sec. 4(2)(f) SREEKUMA 23, HOUSE,NETTISER JOSE East BAILED BY 3 SREEKUMA at 22:40 r/w 5 of BIBIN C V R Male RY,MUKATTUKKA JUNCTION (Thrissur POLICE R Hrs Kerala RA City) Epidemic Diseases Ordinance 2020 3088/2020 U/s 269, 290 IPC & 118(e) of KP Act & Thrissur ADATT 27-06-2020 Sec. 4(2)(f) SOORYAVA 28, JOSE East BAILED BY 4 SOMAN HOUSE,ADATT,TH at 22:25 r/w 5 of BIBIN C V RTHAN Male JUNCTION (Thrissur POLICE RISSUR Hrs Kerala City) Epidemic Diseases Ordinance 2020 386/2020 U/s 118(e) of KP THEKKANATHU( Act & Sec. -
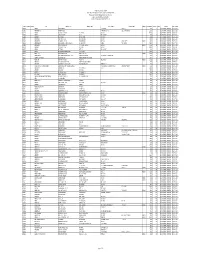
CSBL Unpaid Dividend, Refund Consolidated As on 22.09.2015.Xlsx
The Catholic Syrian Bank Limited Regd. Office, "CSB Bhavan", St. Mary's College Road, Thrissur 680020 Phone: 0487 -2333020, 6451640, eMail: [email protected] List of Unpaid Dividend as on 22.09.2015 (Dividend for the periods 2007-08 to 2013-14) FOLIO / DEMAT ID INITLS NAME ADDRESS LINE 1 ADDRESS LINE 2 ADDRESS LINE 3 ADDRESS LINE 4 PINCOD DIV.AMOUNT DWNO MICR PERIOD IEPF. TR. DATE A00350 ANTONY PALLANS HOUSE KURIACHARA TRICHUR, 30.00 0 2007-08 UNPAID DIVIDEND 25-OCT-2015 A00385 ANNAMMA P X AKKARA HOUSE PANAMKUTTICHIRA OLLUR, TRICHUR DIST 150.00 5 2007-08 UNPAID DIVIDEND 25-OCT-2015 A00398 ANTONY KUTTENCHERY HOUSE HIGH ROAD TRICHUR 1020.00 0 2007-08 UNPAID DIVIDEND 25-OCT-2015 A00406 ANTONY KALLIATH HOUSE OLLUR TRICHUR DIST 27.00 9 2007-08 UNPAID DIVIDEND 25-OCT-2015 A00409 ANTHONY PLOT NO 143 NEHRU NAGAR TRICHUR-6 120.00 0 2007-08 UNPAID DIVIDEND 25-OCT-2015 A00643 ANTHAPPAN PADIKKALA HOUSE EAST FORT GATE TRICHUR 540.00 12 2007-08 UNPAID DIVIDEND 25-OCT-2015 A00647 ANTHONY O K OLAKKENGAL HOUSE LOURDEPURAM TRICHUR - KERALA STATE. 680005 180.00 13 2007-08 UNPAID DIVIDEND 25-OCT-2015 A00668 ANTHONISWAMI C/O INASIMUTHU MUDALIAR SONS 55 NEW STREET KARUR TAMILNADU 2100.00 14 2007-08 UNPAID DIVIDEND 25-OCT-2015 A00822 ANNA JACOB C/O J S MANAVALAN 5 V R NAGAR ADAYAR MADRAS - 600020 210.00 18 2007-08 UNPAID DIVIDEND 25-OCT-2015 A01072 ANTHONY VI/62 PALACE VIEW EAST FORT TRICHUR 4200.00 0 2007-08 UNPAID DIVIDEND 25-OCT-2015 A01077 ANTONY KOTTEKAD KUTTUR TRICHUR DIST 30.00 0 2007-08 UNPAID DIVIDEND 25-OCT-2015 A01103 ANTONY ELUVATHINGAL CHERUVATHERI -

Report of Rapid Impact Assessment of Flood/ Landslides on Biodiversity Focus on Community Perspectives of the Affect on Biodiversity and Ecosystems
IMPACT OF FLOOD/ LANDSLIDES ON BIODIVERSITY COMMUNITY PERSPECTIVES AUGUST 2018 KERALA state BIODIVERSITY board 1 IMPACT OF FLOOD/LANDSLIDES ON BIODIVERSITY - COMMUnity Perspectives August 2018 Editor in Chief Dr S.C. Joshi IFS (Retd) Chairman, Kerala State Biodiversity Board, Thiruvananthapuram Editorial team Dr. V. Balakrishnan Member Secretary, Kerala State Biodiversity Board Dr. Preetha N. Mrs. Mithrambika N. B. Dr. Baiju Lal B. Dr .Pradeep S. Dr . Suresh T. Mrs. Sunitha Menon Typography : Mrs. Ajmi U.R. Design: Shinelal Published by Kerala State Biodiversity Board, Thiruvananthapuram 2 FOREWORD Kerala is the only state in India where Biodiversity Management Committees (BMC) has been constituted in all Panchayats, Municipalities and Corporation way back in 2012. The BMCs of Kerala has also been declared as Environmental watch groups by the Government of Kerala vide GO No 04/13/Envt dated 13.05.2013. In Kerala after the devastating natural disasters of August 2018 Post Disaster Needs Assessment ( PDNA) has been conducted officially by international organizations. The present report of Rapid Impact Assessment of flood/ landslides on Biodiversity focus on community perspectives of the affect on Biodiversity and Ecosystems. It is for the first time in India that such an assessment of impact of natural disasters on Biodiversity was conducted at LSG level and it is a collaborative effort of BMC and Kerala State Biodiversity Board (KSBB). More importantly each of the 187 BMCs who were involved had also outlined the major causes for such an impact as perceived by them and suggested strategies for biodiversity conservation at local level. Being a study conducted by local community all efforts has been made to incorporate practical approaches for prioritizing areas for biodiversity conservation which can be implemented at local level. -

Accused Persons Arrested in Thrissur City District from 14.06.2020To20.06.2020
Accused Persons arrested in Thrissur City district from 14.06.2020to20.06.2020 Name of Name of the Name of the Place at Date & Arresting Court at Sl. Name of the Age & Cr. No & Sec Police father of Address of Accused which Time of Officer, which No. Accused Sex of Law Station Accused Arrested Arrest Rank & accused Designation produced 1 2 3 4 5 6 7 8 9 10 11 579/2020 U/s 188, 269 IPC & 118(e) of PUTHENKADU JAYAPRADE KP Act & Sec. PAZHAYA COLONY , 20-06-2020 EP K G, SI OF JINEESH 27, 4(2)(d) r/w 5 NNUR BAILED BY 1 VASU ELANAD DESOM, ELANAD at 20:05 POLICE VASU Male of Kerala (Thrissur POLICE PAZHAYANNUR Hrs PAZHAYAN Epidemic City) VILLAGE NUR PS Diseases Ordinance 2020 579/2020 U/s 188, 269 IPC MALAMBATHY & 118(e) of JAYAPRADE HOUSE, KP Act & Sec. PAZHAYA 20-06-2020 EP K G, SI OF RADHAKRI 20, THIRUMANI , 4(2)(d) r/w 5 NNUR BAILED BY 2 REJU ELANAD at 20:05 POLICE SHNAN Male ELANAD DESOM, of Kerala (Thrissur POLICE Hrs PAZHAYAN PAZHAYANNUR Epidemic City) NUR PS VILLAGE Diseases Ordinance 2020 579/2020 U/s 188, 269 IPC & 118(e) of THEKKINKADU JAYAPRADE KP Act & Sec. PAZHAYA SANEESH COLONY, 20-06-2020 EP K G, SI OF CHANDRA 31, 4(2)(d) r/w 5 NNUR BAILED BY 3 CHANDRA ELANAD DESOM, ELANAD at 20:05 POLICE N Male of Kerala (Thrissur POLICE N PAZHAYANNUR Hrs PAZHAYAN Epidemic City) VILLAGE NUR PS Diseases Ordinance 2020 677/2020 U/s 269 IPC & JAYACHAN 118(e) of KP DRAN.K.M, Act & Sec. -

Accused Persons Arrested in Thrissur City District from 15.07.2018 to 21.07.2018
Accused Persons arrested in Thrissur City district from 15.07.2018 to 21.07.2018 Name of Name of the Name of the Place at Date & Arresting Court at Sl. Name of the Age & Cr. No & Sec Police father of Address of Accused which Time of Officer, which No. Accused Sex of Law Station Accused Arrested Arrest Rank & accused Designation produced 1 2 3 4 5 6 7 8 9 10 11 SATHEESH NEDUPUZ MALAYATH 21-07-2018 KUMAR, SI PARAMESW 45, SAKTHAN 450/2018 U/s HA 1 SUNDARAN (H),OORAKKAD,K at 23:20 OF POLICE, ARRESTED ARAN NAIR Male NAGAR 151 CrPC (THRISSUR UNDUKKAD Hrs NEDUPUZH CITY) A PS SATHEESH NELLISSERY NEDUPUZ 21-07-2018 KUMAR, SI 39, (H),PANNITHADA SAKTHAN 450/2018 U/s HA 2 LINSAN WILSON at 23:20 OF POLICE, ARRESTED Male M,KUNNAMKULA NAGAR 151 CrPC (THRISSUR Hrs NEDUPUZH M CITY) A PS VAKAYIL HOUSE,PO SATHEESH NEDUPUZ NEDUPUZHA,PAN 21-07-2018 449/2018 U/s KUMAR, SI 33, KOORKKEN HA BAILED BY 3 SANDEEP VENU AMUKKU,NEAR at 22:00 279 IPC & 185 OF POLICE, Male CHERY (THRISSUR POLICE THRITHAMARASS Hrs MV ACT NEDUPUZH CITY) ERY A PS TEMBLE,THRISSUR PADINJARE ANTHOOR HOUSE, WADAKKA 21-07-2018 401/2018 U/s MURALEED RAMANKU 49, VITHANASSERY PUNNAMPA NCHERRY K C BAILED BY 4 at 20:20 118(a) of KP HARAN TTY NAIR Male DESOM, RAMBU (THRISSUR RATHEESH POLICE Hrs Act VALLANGY CITY) VILLAGE, PALAKKAD ANGADIPARAMBI GURUVAY L HOUSE 21-07-2018 520/2018 U/s 34, UR ANUDAS K, BAILED BY 5 SREEJESH KRISHNAN P.O.KAKKASSERY THAIKKAD at 20:00 279 IPC & 185 Male (THRISSUR SI OF PLOCE POLICE ELAVALLY Hrs MV ACT CITY) VILLAGE CHAKKENDAN 21-07-2018 TRAFFIC NANDAKU SUDHAKAR 41, MATHRUBH -

Ac Name Ac Addr1 Ac Addr2 Ac Addr3 Sunny Joseph Alias Varghese K Elayadathukudy House Thazhathoor Po, Kozhuvanawynad673595
AC_NAME AC_ADDR1 AC_ADDR2 AC_ADDR3 SUNNY JOSEPH ALIAS VARGHESE K ELAYADATHUKUDY HOUSE THAZHATHOOR P O, KOZHUVANAWYNAD673595 (VARGHESE K J) S T RAJALINGAM 18 E.M.M PILLAI-111 STREET CHENNIMALAI ROAD ERODE-638001 MINI JACOB MULLASSERIL HOUSE KADAMPANAD NORTH SANJU P R PANAYAMTHONDALIL THUVAYOOOR NORTH P O NELLIMUKAL NIJO MIGHEL KODIYAN HOUSE SANKARAIYER ROAD TRICHUR JOSEPH A V CHIEF MANAGER CSB,KOTTAYAM MOHANAN NAIR V AITTY KONAM M J BHAVAN THURUVICKAL P O MEDICAL COLLEGE PO TRIVANDRUM SURENDRAN V U S/O UNNIKRISHNAN VENKITANGU HOUSE CHETTUPUZHA ANUPA JOHN EMMATTY HOUSE GILGAL STREET KURA NELLIKKUNNU TRISSUR RAGUVARAN R 91,GANDHI NAGAR PALLAVAN SALAI CHENNAI 2 DINU K U KUNJALAKATTU HOUSE KAPPINCHAL CHERUKATTOOR P O JECCO ANTONY ROSE BHAVAN SOUTH KEERTHI NAGAR ROAD COCHIN 26 SAINUDHEEN KANJIRAMADATHIL HOUSE P.O.THOZHUVANNUR VIA.VALANCHERY SIMON CHERIAN 31.4TH CROSS JAI BHARATH NAGAR BANGALORE.560033 SHINNY SHAJI W/O SHAJI THOMAS KIZHAKKE PALANTHARA ERAVIPEROOR P.O. KASUMANI V S/O VELAN AKAMPADAM, POTHUNDY NEMMARA-678508 REJI K PETER KUDILIL HOUSE ENKAKAD P O ,MANGARA WADAKANCHERY SATHEESH R S/O RAMACHANDRAN KAIPPION KOLOUMB NANNIODE P O SAJEEVKUMAR S V S/O SASIKUMAR TC 14/1410,VIGNESWARA NAGAR,15,VAZHUTHACAUD,TRIVANDRUM DILEEP B PRADEEP MANDIRAM PUNTHALATHAZHAM KILIKOLLUR PO KOLLAM RAJAMMA K K LAKSHAM VEEDU-10 THONAKKAD P O CHERIYANAD BIJU M MOHANALAYAM PELA PO MAVELIKKAR-3 DR MOHAMED RAFEEQ K A KIZHAKKEY VEETIL EDAVILANGU P OITAL KODUNGALLUR THRISSUR DIST GEORGE JOSEPH MOONUTHOTTIYIL HOUSE VADAKKANIRAPPU NEEZHOOR RAMTIRATH V TIWARI SHIV SADAN BULDG, B-WING,1ST FLOOR,B-BULDG KATEMANIVALI,KALYAN,THANE-DT VEENA KUDUNBASREE WARD NO 2 PANDANAD PANCHAYATH PANDANAD PO LATHA 2199,III CROSS BASWESHWARA ROAD, MYSORE RAHIL LAKRA NEW DELHI 1 CH VISHNUVARDHAN 18-10-16,4TH LANE KEDARESWAPET,WARD NO 50 VIJAYAWADA-520002 RAJU.M. -

Hymenoptera: Vespidae: Polistinae) from South India
Biological Forum Forum —– AnAn InternationalInternational Journal, Journal4,(2):1(1): 8-9(2012)12 -17 (2009) ISSN No. (Print) : 0975-1130 ISSN No. (Online) : 2249-3239 New Record of Polistes (Polistella) strigosus Bequaert (Hymenoptera: Vespidae: Polistinae) from South India Lambert Kishore, K.P. Mohammed Shareef and P. Girish Kumar* Malabar Christian College, Kozhikode, Kerala *Zoological Survey of India, New Alipore, Kolkata, (W.B.) (Received 23 , 2012, Accepted 25 May 2012) ABSTRACT : Polistes (Polistella) strigosus Bequaert is herewith recorded for the first time from South India and also from Western Ghats. Keywords : Polistes (Polistella) strigosus Bequaert, Western Ghats, Kerala, South India, new record. INTRODUCTION Das and Gupta and P. (P.) strigosus mimus Bequaert are recorded from Indian subcontinent of which P. (P.) strigosus The genus Polistes Latrielle is the cosmopolitan genus atratus Das and Gupta is reported from India (Assam, Bihar, which is most abundant and widely distributed among social Delhi, Manipur, Sikkim, Tripura, Uttarakhand and West Vespidae. They are commonly known as paper wasps and Bengal). None of them were reported from South India. The usually make relatively small colonies and usually build their black and brown colour pattern is highly variable in this nests in human inhabited areas. They are generally non- species. It requires further studies with more specimens aggressive compared to other social wasps but can be from different localities for confirming the status of different provoked into an aggressive morale for defending their colour variants (subspecies). So, at present, we are not nests. They are considered as beneficial insects since all dealing with the colour variants (subspecies) of this species the species are predatory and many consume large numbers here. -

Acinetobacter Sp
1 Acinetobacter sp. - An acid-tolerant phosphate solubilizing bacteria from lateritic soils Athulya, M.M., K.Surendra Gopal* and D.Girija Department of Agricultural Microbiology, College of Horticulture, Kerala Agricultural University, Vellanikkara, Thrissur-680656 Corresponding author: [email protected] Abstract A study was conducted to screen the isolates of phosphate solubilizing bacteria (PSB) ls, for efficiency and acid tolerance. The rhizosphere and non-rhizosphere soil samples were collected from various lateritic soils in Thrissur district of Kerala and phosphate solubilizing bacteria were isolated on Pikovskaya’s agar medium. Under in vitro conditions, the bacterial isolate PMD-7 (Acinetobacter sp.) solubilized 207.2 µg/ml of insoluble phosphorus. Its solubilization efficiency in Pikovskaya’s agar medium was 247.62%. It grew in the pH range of 4.0 to 7.0 and tolerated upto pH 3.5. When it was grown in Pikovskaya’s broth, it showed a reduction in pH from pH 7.0 to 4.35 indicating the production of organic acids, which is one of the mechanism involved in ‘P’ solubilization by microbes. The bacterial isolate also produced 33µg/ml of indole acetic acid (IAA) which is a phytohormone for the plant growth. The molecular characterization using 16s rDNA sequencing revealed the identity of bacteria as Acinetobacter sp. However, this new acid-tolerant PSB is the first report from Kerala, which could be a potential biofertilizer for acidic soils of Kerala. Key words: Acinetobacter sp., phosphate solubilizing bacteria, acid-tolerant, lateritic soil Introduction Phosphorus is one of the essential macronutrients for plants and is applied to soil in the form of phosphatic fertilizers.