Acinetobacter Sp
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Accused Persons Arrested in Thrissur City District from 12.06.2016 to 18.06.2016
Accused Persons arrested in Thrissur City district from 12.06.2016 to 18.06.2016 Name of Name of the Name of the Place at Date & Arresting Court at Sl. Name of the Age & Cr. No & Sec Police father of Address of Accused which Time of Officer, which No. Accused Sex of Law Station Accused Arrested Arrest Rank & accused Designation produced 1 2 3 4 5 6 7 8 9 10 11 KOLAPPULLY HOUSE, 2078/16 U/S TOWN EAST 12.06.2016 M K AJAYAN, SI BAILED BY 1 RAGESH K R RAJAN 29 MALE MULAYAM P O, DIVANJIMOOLA 15(C) R/W 63 PS (THRISSUR at 00.15 OF POLICE POLICE VALAKKAVU ABKARAI ACT CITY) AMBATT HOUSE, 2079/16 U/S TOWN EAST 12.06.2016 M K AJAYAN, SI BAILED BY 2 VARGHESE A T THOMAS 46 MALE MULAYAM P O , DIVANJIMOOLA 15(C) R/W 63 PS (THRISSUR at 00.22 OF POLICE POLICE VALAKKAVU ABKARAI ACT CITY) MELAYIL HOUSE, 2080/16 U/S TOWN EAST RAMACHAND 12.06.2016 M K AJAYAN, SI BAILED BY 3 RAMAN 47 MALE MULAYAM P O , DIVANJIMOOLA 15(C) R/W 63 PS (THRISSUR RAN at 00.30 OF POLICE POLICE VALAKKAVU ABKARAI ACT CITY) MULLOOKKARAN 2081/16 U/S T OWN EAST 12.06.2016 M.K. AJAYAN, BAILED BY 4 SHIJI RAPPAI 39 MALE HOUSE, MULAYAM DIVANJIMOOLA 15(C) R/W 63 PS (THRISSUR AT 00.29 SI OF POLICE POLICE VALAKKAVU ABKARAI ACT CITY) PALUKKASSERY 2082/16 U/S TOWN EAST CHANDRASEKH 12.06.2016 M K AJAYAN, SI BAILED BY 5 RAJKUMAR 48 MALE HOUSE, MULAYAM P DIVANJIMOOLA 15(C) R/W 63 PS (THRISSUR ARAN at 00.50 OF POLICE POLICE O , VALAKKAVU ABKARAI ACT CITY) THACHATTIL HOUSE,NEAR 2084/16 U/S TOWN EAST V.K. -

List of Teachers Posted from the Following Schools to Various Examination Centers As Assistant Superintendents for Higher Secondary Exam March 2015
LIST OF TEACHERS POSTED FROM THE FOLLOWING SCHOOLS TO VARIOUS EXAMINATION CENTERS AS ASSISTANT SUPERINTENDENTS FOR HIGHER SECONDARY EXAM MARCH 2015 08001 - GOVT SMT HSS,CHELAKKARA,THRISSUR 1 DILEEP KUMAR P V 08015-GOVT HSS,CHERUTHURUTHY,THRISSUR 04884231495, 9495222963 2 SWAPNA P 08015-GOVT HSS,CHERUTHURUTHY,THRISSUR , 9846374117 3 SHAHINA.K 08035-GOVT. RSR VHSS, VELUR, THRISSUR 04885241085, 9447751409 4 SEENA M 08041-GOVT HSS,PAZHAYANNOOR,THRISSUR 04884254389, 9447674312 5 SEENA P.R 08046-AKM HSS,POOCHATTY,THRISSUR 04872356188, 9947088692 6 BINDHU C 08062-ST ANTONY S HSS,PUDUKAD,THRISSUR 04842331819, 9961991555 7 SINDHU K 08137-GOVT. MODEL HSS FOR GIRLS, THRISSUR TOWN, , 9037873800 THRISSUR 8 SREEDEVI.S 08015-GOVT HSS,CHERUTHURUTHY,THRISSUR , 9020409594 9 RADHIKA.R 08015-GOVT HSS,CHERUTHURUTHY,THRISSUR 04742552608, 9847122431 10 VINOD P 08015-GOVT HSS,CHERUTHURUTHY,THRISSUR , 9446146634 11 LATHIKADEVI L A 08015-GOVT HSS,CHERUTHURUTHY,THRISSUR 04742482838, 9048923857 12 REJEESH KUMAR.V 08015-GOVT HSS,CHERUTHURUTHY,THRISSUR 04762831245, 9447986101 08002 - GOVT HSS,CHERPU,THRISSUR 1 PREETHY M K 08003-GOVT MODEL GHSS, IRINJALAKKUDA, THRISSUR 04802820505, 9496288495 2 RADHIKA C S 08003-GOVT MODEL GHSS, IRINJALAKKUDA, THRISSUR , 9495853650 3 THRESSIA A.O 08005-GOVT HSS,KODAKARA,THRISSUR 04802726280, 9048784499 4 SMITHA M.K 08046-AKM HSS,POOCHATTY,THRISSUR 04872317979, 8547619054 5 RADHA M.R 08050-ST ANTONY S HSS,AMMADAM,THRISSUR 04872342425, 9497180518 6 JANITHA K 08050-ST ANTONY S HSS,AMMADAM,THRISSUR 04872448686, 9744670871 1 7 SREELEKHA.E.S 08050-ST ANTONY S HSS,AMMADAM,THRISSUR 04872343515, 9446541276 8 APINDAS T T 08095-ST. PAULS CONVENT EHSS KURIACHIRA, THRISSUR, 04872342644, 9446627146 680006 9 M.JAMILA BEEVI 08107-SN GHSS, KANIMANGALAM, THRISSUR, 680027 , 9388553667 10 MANJULA V R 08118-TECHNICAL HSS, VARADIAM, THRISSUR, 680547 04872216227, 9446417919 11 BETSY C V 08138-GOVT. -
![Ticf Kkddv KERALA GAZETTE B[Nimcniambn {]Kn≤S∏Spøp∂Xv PUBLISHED by AUTHORITY](https://docslib.b-cdn.net/cover/9289/ticf-kkddv-kerala-gazette-b-nimcniambn-kn-s-sp%C3%B8p-xv-published-by-authority-329289.webp)
Ticf Kkddv KERALA GAZETTE B[Nimcniambn {]Kn≤S∏Spøp∂Xv PUBLISHED by AUTHORITY
© Regn. No. KERBIL/2012/45073 tIcf k¿°m¿ dated 5-9-2012 with RNI Government of Kerala Reg. No. KL/TV(N)/634/2015-17 2016 tIcf Kkddv KERALA GAZETTE B[nImcnIambn {]kn≤s∏SpØp∂Xv PUBLISHED BY AUTHORITY 2016 Pq¨ 14 Xncph\¥]pcw, hmeyw 5 14th June 2016 \º¿ sNmΔ 1191 CShw 31 31st Idavam 1191 24 Vol. V } Thiruvananthapuram, No. } 1938 tPyjvTw 24 Tuesday 24th Jyaishta 1938 PART IB Notifications and Orders issued by the Kerala Public Service Commission NOTIFICATIONS (2) (1) No. Estt.III(1)35557/03/GW. Thiruvananthapuram, 10th May 2016. No. Estt.III(1)35150/03/GW. Thiruvananthapuram, 10th May 2016. The following is the list of Deputy Secretaries found The following is the list of Joint Secretaries found fit fit by the Departmental Promotion Committee and by the Departmental Promotion Committee and approved approved by the Kerala Public Service Commission for by the Kerala Public Service Commission for promotion to promotion to the post of Joint Secretary/Regional Officer the post of Additional Secretary in the Office of the in the Office of the Kerala Public Service Commission for Kerala Public Service Commission for the year 2016. the year 2016. 1. Sri Ramesh Sarma, P. 1. Smt. Sheela Das 2. Sri Thomas M. Mathew 2. Sri Ganesan, K. 3. Smt. Vijayamma, P. R. 3. Sri Sandeep, N. The above list involves no supersession. The above list involves no supersession. 21 14th JUNE 2016] KERALA GAZETTE 586 (3) NOTIFICATION No. Estt.III(1)35933/03/GW. No. Estt.III(1)36207/03/GW. Thiruvananthapuram, 10th May 2016. -

Accused Persons Arrested in Thrissur City District from 25.08.2019To31.08.2019
Accused Persons arrested in Thrissur City district from 25.08.2019to31.08.2019 Name of Name of the Name of the Place at Date & Arresting Court at Sl. Name of the Age & Cr. No & Sec Police father of Address of Accused which Time of Officer, which No. Accused Sex of Law Station Accused Arrested Arrest Rank & accused Designation produced 1 2 3 4 5 6 7 8 9 10 11 VADAPARAMBIL Thrissur HOUSE, 31-08-2019 979/2019 U/s 34, KSRTC BUS East BIBIN, SI OF BAILED BY 1 ANOOP HARI KOLOTHUPADI, at 02:00 279 IPC & 185 Male STAND (Thrissur POLICE POLICE GANDHIGRAMAM Hrs MV ACT City) , IRIJALAKUDA HOPE COTTEGE, SREEJITH.D, 31-08-2019 Viyyur IN JOHN 39, THAYATTUPARA 767/2019 U/s SI OF 2 SHIJU JOHN VIYYUR at 11:30 (Thrissur JUDICIAL DAVIS Male MBU, KOTTULI, 379 IPC POLICE, Hrs City) CUSTODY KOZHIKODE. VIYYUR PS THALAYKATTU Anandha 30-08-2019 235/2019 U/s GVR Temple RAMANAT 45, HOUSE, MANJULAL Krishnan A , BAILED BY 3 SANTHOSH at 20:10 279 IPC & 185 (Thrissur HAN Male MAMMIYYOOR, JUNCTION SI of Police, POLICE Hrs MV ACT City) GURUVAYUR Temple PS KARUTHEDATH 30-08-2019 417/2019 U/s Pavaratty RADHAKRI 40, HOUSE, PAVARATTY BAILED BY 4 PRAJEESH at 21:30 279 IPC & 185 (Thrissur SI JOSEPH SHNAN Male KOOTTANAD, PS POLICE Hrs MV ACT City) PALAKKAD 30-08-2019 537/2019 U/s CHAVAKA 50, Thodu house Blangad BAILED BY 5 Ravi Kunju velayi at 19:54 279 IPC & 185 D (Thrissur SI Anand kp Male blangad chavakkad chavakkad POLICE Hrs MV ACT City) Chetti theruvu PAZHAYA E Babu ,si of Kattukulam,T 30-08-2019 383/2019 U/s 29, house NNUR police BAILED BY 6 SURESH MURUKAN hiruvilwamal -

KFRI-RR240.Pdf
STUDY OF SOCIAL AND ECONOMIC DEPENDENCIES OF THE LOCAL COMMUNITIES ON PROTECTED AREAS - A CASE OF PEECHI-VAZHANI AND CHIMMONI WILDLIFE SANCTUARIES Final Report of Project KFRI / 352 / 2000 V Anitha P.K.Muraleedharan Division of Forest Economics A project sponsored by KERALA FOREST DEPARTMENT (WBP) Kerala Forest Research Institute, Peechi, Thrissur - 680 653, Kerala July 2002 ABSTRACT OF PROJECT PROPOSAL 1. Project number : KFRI 352/2000 2. Title of the project : Study of Social and economic dependencies of local communities on Protected Areas : A case of Peechi- Vazhani and Chimmoni Wildlife Sanctuaries. 3. Objective : 1. Survey on the economic and social status of local communities in the Protected Areas and classification of the local communities based on their dependencies on the Protected Areas. 2. Quantification and classification of NTFP collected from the Protected Areas by the local communities (both legal and illegal). 3. Assessment of grazing pressure on the Protected Areas. 4. Enumerations of other human-related problems affecting conservation and management of the study areas and evolve suitable management strategies through a workshop with the participation of local community representatives and forest officials. 5. Assessing the level of conservation awareness among the local people and their attitude towards conservation activities by the Department 6. Suggest scientific methods for the collection of various important NTFPs and methods for their value-addition (based on literature). 7. Develop a training programme for the local people for scientific collection of NTFP for its sustainability and value-addition. 1. Quantify the level of socio-economic 4. Expected outcome : dependencies of the local communities on the sanctuary 2. -

Of Thrissur City Sl
Weekly Consolidated list of arrested persons (22.02.2015 to 28.02.2015) of Thrissur City Sl. Name of the Name of the Father of Age & Address of accused Place at which Date & Time of Crim No. & Sec of Police Station Name of Arresting Name of the No Accused Accused Sex arrested Arrest Law Officer Court at which accused produced 1 Ranjith Raghavan 33 Male Arakkal House, Athekkad Athekkad 23.02.2015 Cr. 246/15 U/s 151 Viyyur K. K. Mohandas, SI of JFCM I, Thrissur Desom, Kolazhy Village at 09.00 Hrs CrPC Police, Viyyur 2 Sanesh Chandran 31 Male Chettaltamveetil House, Ayyanthole 24-02-2015 Cr. 245/15 U/s 408, Thrissur West P. R. Bejoy, SI of CJM Court, Chombala P.o, Vadakkara at 14.30 Hrs 418, 420 IPC Police, Thrissur West Thrissur Kozhikode 3 Lijo Poulouse 28 Male Thundiyil Veedu, Thamburattimoola 24.02.2015 Cr. 45/15 u/s 461, 457, Ollur M. G. Ponnappan, SI JFCM III, Thrissur Thamburattimoola, Puthur at 08.00 Hrs 380 IPC of Police, Ollur 4 Ranjith Unnimon 23 Male Elembalamkattil House, Peramangalam 24-02-2015 Cr. 280/15 U/s 366(a), Peramangalam T. N. Sudhakaran, SI JFCM, Kuzhikkattukonnam, at 10.00 Hrs 376 IPC & 3, 4, 5 of of Police, Kunnamkulam Madayikonnam POCSO Act-2012 Peramangalam 5 Ratheesh Sudhakaran 33 Male Kadavarath House, Peramangalam 24-02-2015 Cr. 292/15 U/s 447, Peramangalam T. N. Sudhakaran, SI JFCM, Ambedkar Nagar, Avanur at 09.00 hrs 326, 506(ii) IPC of Police, Kunnamkulam Peramangalam 6 Aneesh Anandan 28 Male Aranattil (H) Aloor (V) Guruvayur 25.02.2015 Cr.202/15 U/s 341 323 Guruvayur M Sasidharan, SI of JFCM, Chavakkad -
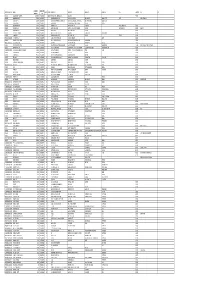
Mgl-Di219-Unpaid Share Holders List As on 31-03-2020
DIVIDEND WARRANT FOLIO-DEMAT ID NAME MICR DDNO ADDRESS 1 ADDRESS 2 ADDRESS 3 ADDRESS 4 CITY PINCODE JH1 JH2 AMOUNT NO 001221 DWARKA NATH ACHARYA 220000.00 192000030 680109 5 JAG BANDHU BORAL LANE CALCUTTA 700007 000642 JNANAPRAKASH P.S. 2200.00 192000034 18 POZHEKKADAVIL HOUSE P.O.KARAYAVATTAM TRICHUR DIST. KERALA STATE 68056 MRS. LATHA M.V. 000691 BHARGAVI V.R. 2200.00 192000035 19 C/O K.C.VISHWAMBARAN,P.B.NO.63 ADV.KAYCEE & KAYCEE AYYANTHOLE TRICHUR DISTRICT KERALA STATE 002679 NARAYANAN P S 2200.00 192000051 35 PANAT HOUSE P O KARAYAVATTOM, VALAPAD THRISSUR KERALA 002976 VIJAYA RAGHAVAN 2200.00 192000056 40 KIZHAKAYIL (H) KEEZHARIYUR P O KOVILANDY KHARRUNNISSA P M 000000 003124 VENUGOPAL M R 2200.00 192000057 41 MOOTHEDATH (H) SAWMILL ROAD KOORVENCHERY THRISSUR GEETHADEVI M V 000000 RISHI M.V. 003292 SURENDARAN K K 2068.00 192000060 44 KOOTTALA (H) PO KOOKKENCHERY THRISSUR 000000 003442 POOKOOYA THANGAL 2068.00 192000063 47 MECHITHODATHIL HOUSE VELLORE PO POOKOTTOR MALAPPURAM 000000 003445 CHINNAN P P 2200.00 192000064 48 PARAVALLAPPIL HOUSE KUNNAMKULAM THRISSUR PETER P C 000000 IN30611420024859 PUSHPA DEVI JAIN 2750.00 192000075 59 A-402, JAWAHAR ENCLAVE JAWAHAR NAGAR JAIPUR 302004 001431 JITENDRA DATTA MISRA 6600.00 192000079 63 BHRATI AJAY TENAMENTS 5 VASTRAL RAOD WADODHAV PO AHMEDABAD 382415 IN30177410163576 Rukaiya Kirit Joshi 2695.00 192000098 82 303 Anand Shradhanand Road Vile Parle East Mumbai 400057 000493 RATHI PRATAP POYYARA 2200.00 192000101 85 10,GREENVILLA,NETAJIPALKARMARG GHATKOPAR(WEST) MUMBAI MAHARASTRA 400084 MR. PRATAP APPUNNY POYYARA 001012 SHARAVATHY C.H. 2200.00 192000102 86 W/O H.L.SITARAMAN, 15/2A,NAV MUNJAL NAGAR,HOUSING CO-OPERATIVE SOCIETY CHEMBUR, MUMBAI 400089 1201090700097429 NANASAHEB BALIRAM SONAWANE 1606.00 192000114 98 2 PALLAWI HSG SOC. -

Report of Rapid Impact Assessment of Flood/ Landslides on Biodiversity Focus on Community Perspectives of the Affect on Biodiversity and Ecosystems
IMPACT OF FLOOD/ LANDSLIDES ON BIODIVERSITY COMMUNITY PERSPECTIVES AUGUST 2018 KERALA state BIODIVERSITY board 1 IMPACT OF FLOOD/LANDSLIDES ON BIODIVERSITY - COMMUnity Perspectives August 2018 Editor in Chief Dr S.C. Joshi IFS (Retd) Chairman, Kerala State Biodiversity Board, Thiruvananthapuram Editorial team Dr. V. Balakrishnan Member Secretary, Kerala State Biodiversity Board Dr. Preetha N. Mrs. Mithrambika N. B. Dr. Baiju Lal B. Dr .Pradeep S. Dr . Suresh T. Mrs. Sunitha Menon Typography : Mrs. Ajmi U.R. Design: Shinelal Published by Kerala State Biodiversity Board, Thiruvananthapuram 2 FOREWORD Kerala is the only state in India where Biodiversity Management Committees (BMC) has been constituted in all Panchayats, Municipalities and Corporation way back in 2012. The BMCs of Kerala has also been declared as Environmental watch groups by the Government of Kerala vide GO No 04/13/Envt dated 13.05.2013. In Kerala after the devastating natural disasters of August 2018 Post Disaster Needs Assessment ( PDNA) has been conducted officially by international organizations. The present report of Rapid Impact Assessment of flood/ landslides on Biodiversity focus on community perspectives of the affect on Biodiversity and Ecosystems. It is for the first time in India that such an assessment of impact of natural disasters on Biodiversity was conducted at LSG level and it is a collaborative effort of BMC and Kerala State Biodiversity Board (KSBB). More importantly each of the 187 BMCs who were involved had also outlined the major causes for such an impact as perceived by them and suggested strategies for biodiversity conservation at local level. Being a study conducted by local community all efforts has been made to incorporate practical approaches for prioritizing areas for biodiversity conservation which can be implemented at local level. -

Accused Persons Arrested in Thrissur City District from 14.06.2020To20.06.2020
Accused Persons arrested in Thrissur City district from 14.06.2020to20.06.2020 Name of Name of the Name of the Place at Date & Arresting Court at Sl. Name of the Age & Cr. No & Sec Police father of Address of Accused which Time of Officer, which No. Accused Sex of Law Station Accused Arrested Arrest Rank & accused Designation produced 1 2 3 4 5 6 7 8 9 10 11 579/2020 U/s 188, 269 IPC & 118(e) of PUTHENKADU JAYAPRADE KP Act & Sec. PAZHAYA COLONY , 20-06-2020 EP K G, SI OF JINEESH 27, 4(2)(d) r/w 5 NNUR BAILED BY 1 VASU ELANAD DESOM, ELANAD at 20:05 POLICE VASU Male of Kerala (Thrissur POLICE PAZHAYANNUR Hrs PAZHAYAN Epidemic City) VILLAGE NUR PS Diseases Ordinance 2020 579/2020 U/s 188, 269 IPC MALAMBATHY & 118(e) of JAYAPRADE HOUSE, KP Act & Sec. PAZHAYA 20-06-2020 EP K G, SI OF RADHAKRI 20, THIRUMANI , 4(2)(d) r/w 5 NNUR BAILED BY 2 REJU ELANAD at 20:05 POLICE SHNAN Male ELANAD DESOM, of Kerala (Thrissur POLICE Hrs PAZHAYAN PAZHAYANNUR Epidemic City) NUR PS VILLAGE Diseases Ordinance 2020 579/2020 U/s 188, 269 IPC & 118(e) of THEKKINKADU JAYAPRADE KP Act & Sec. PAZHAYA SANEESH COLONY, 20-06-2020 EP K G, SI OF CHANDRA 31, 4(2)(d) r/w 5 NNUR BAILED BY 3 CHANDRA ELANAD DESOM, ELANAD at 20:05 POLICE N Male of Kerala (Thrissur POLICE N PAZHAYANNUR Hrs PAZHAYAN Epidemic City) VILLAGE NUR PS Diseases Ordinance 2020 677/2020 U/s 269 IPC & JAYACHAN 118(e) of KP DRAN.K.M, Act & Sec. -

Ac Name Ac Addr1 Ac Addr2 Ac Addr3 Sunny Joseph Alias Varghese K Elayadathukudy House Thazhathoor Po, Kozhuvanawynad673595
AC_NAME AC_ADDR1 AC_ADDR2 AC_ADDR3 SUNNY JOSEPH ALIAS VARGHESE K ELAYADATHUKUDY HOUSE THAZHATHOOR P O, KOZHUVANAWYNAD673595 (VARGHESE K J) S T RAJALINGAM 18 E.M.M PILLAI-111 STREET CHENNIMALAI ROAD ERODE-638001 MINI JACOB MULLASSERIL HOUSE KADAMPANAD NORTH SANJU P R PANAYAMTHONDALIL THUVAYOOOR NORTH P O NELLIMUKAL NIJO MIGHEL KODIYAN HOUSE SANKARAIYER ROAD TRICHUR JOSEPH A V CHIEF MANAGER CSB,KOTTAYAM MOHANAN NAIR V AITTY KONAM M J BHAVAN THURUVICKAL P O MEDICAL COLLEGE PO TRIVANDRUM SURENDRAN V U S/O UNNIKRISHNAN VENKITANGU HOUSE CHETTUPUZHA ANUPA JOHN EMMATTY HOUSE GILGAL STREET KURA NELLIKKUNNU TRISSUR RAGUVARAN R 91,GANDHI NAGAR PALLAVAN SALAI CHENNAI 2 DINU K U KUNJALAKATTU HOUSE KAPPINCHAL CHERUKATTOOR P O JECCO ANTONY ROSE BHAVAN SOUTH KEERTHI NAGAR ROAD COCHIN 26 SAINUDHEEN KANJIRAMADATHIL HOUSE P.O.THOZHUVANNUR VIA.VALANCHERY SIMON CHERIAN 31.4TH CROSS JAI BHARATH NAGAR BANGALORE.560033 SHINNY SHAJI W/O SHAJI THOMAS KIZHAKKE PALANTHARA ERAVIPEROOR P.O. KASUMANI V S/O VELAN AKAMPADAM, POTHUNDY NEMMARA-678508 REJI K PETER KUDILIL HOUSE ENKAKAD P O ,MANGARA WADAKANCHERY SATHEESH R S/O RAMACHANDRAN KAIPPION KOLOUMB NANNIODE P O SAJEEVKUMAR S V S/O SASIKUMAR TC 14/1410,VIGNESWARA NAGAR,15,VAZHUTHACAUD,TRIVANDRUM DILEEP B PRADEEP MANDIRAM PUNTHALATHAZHAM KILIKOLLUR PO KOLLAM RAJAMMA K K LAKSHAM VEEDU-10 THONAKKAD P O CHERIYANAD BIJU M MOHANALAYAM PELA PO MAVELIKKAR-3 DR MOHAMED RAFEEQ K A KIZHAKKEY VEETIL EDAVILANGU P OITAL KODUNGALLUR THRISSUR DIST GEORGE JOSEPH MOONUTHOTTIYIL HOUSE VADAKKANIRAPPU NEEZHOOR RAMTIRATH V TIWARI SHIV SADAN BULDG, B-WING,1ST FLOOR,B-BULDG KATEMANIVALI,KALYAN,THANE-DT VEENA KUDUNBASREE WARD NO 2 PANDANAD PANCHAYATH PANDANAD PO LATHA 2199,III CROSS BASWESHWARA ROAD, MYSORE RAHIL LAKRA NEW DELHI 1 CH VISHNUVARDHAN 18-10-16,4TH LANE KEDARESWAPET,WARD NO 50 VIJAYAWADA-520002 RAJU.M. -

No Name School Address Residence Address Photo 1 Ajitha K National Hss, Irinjalakuda, Ph: 0480- 2821248 Sopanam', Pattamali
NO NAME SCHOOL ADDRESS RESIDENCE ADDRESS PHOTO NATIONAL HSS, SOPANAM', PATTAMALI ROAD, 1 AJITHA K IRINJALAKUDA, PH: 0480- IRINJALAKUDA, 0480-2856444, 2821248 9400163990 ST.CLARE'S CGHSS,TCR, W/O K.M FRANKLIN, KOOLA(H), 2 ANIT P ANTONY 2334432, ANCHAGADY, P.O ARANATTUKARA, [email protected] 9446768220 ILLATHU, KALLIPADAM P.O, KULLAPULLY, GVHSS FOR DEAF, 3 ANITHA K.G SHORNUR, 9447334579, 9447334579, KUNNAMKULAM [email protected] ANITHA KNM VHSS, VATANAPPALLY, KELAMKANDATH HOUSE,ANTHIKAD, 4 MUKUNDAN 0487-2290030 0487-2631938, 8129267675 GHSS ERUMAPETTY, ARIYARATH HOUSE, KIZHOOR, 5 ANJANA C.A ERUMAPETTY,THRISSUR, KUNNAMKULAM, 04885-228366, 04885-264239 9645374280, [email protected] MES HSS, KOLLEZHUTH (H), P.O VEMBALLOOR- SREENARAYANAPURAM, PH: 6 ANJU K.K 680671, 0480-2851924, 9447970105, 0480-2854263, [email protected] [email protected] CHALDEAN SYRIAN HSS,TCR, CHIRAYATH,VAKAYIL ANNE SWAPNA 0487-2422791, ROAD,CHIYYARAM,TCR, 04872250709, 7 MATHEW chaldeansyrianhsstrichur@ya 9447437030,anneswapnamathew@gmail hoo.com .com GHSS AYYATHOLE, KARIAT D4A11KSHB, PULLAZHI P.O, AYYATHOLE P.O ,THRISSUR, 8 ARIFA K M THRISSUR, 680012, PH: 0487-2364218, 680003, PH: 0487-2363437, 9446143474, [email protected] [email protected] ERASSERY(H),EDAMUTTAM- GOVT.GIRLS HSS, 680568,THRISSUR(DT),0480- 9 BABY C.K KODUNGALLUR, 0480- 2835745,9446145598, 2812580 [email protected] MANJALY HOUSE,P.O PUDUKAD,TSR- ST.ANTONY'S 680301,0480- 10 BEBY HELEN HSS,PUDUKAD,0480-2755242 2758516,9497740001,bbmanjaly@yahoo. com HSS PANANGAD,P.O CHEEREPARAMBIL(H), -

Accused Persons Arrested in Thrissur City District from 29.12.2019To04.01.2020
Accused Persons arrested in Thrissur City district from 29.12.2019to04.01.2020 Name of Name of the Name of the Place at Date & Arresting Court at Sl. Name of the Age & Cr. No & Sec Police father of Address of Accused which Time of Officer, which No. Accused Sex of Law Station Accused Arrested Arrest Rank & accused Designation produced 1 2 3 4 5 6 7 8 9 10 11 PANTHAYIL 04-01-2020 8/2020 U/s Guruvayur CHANDRA 32, GURUVAYO K C BAILED BY 1 SALISH HOUSE, at 10:00 279 IPC & 185 (Thrissur N Male OR RATHEES POLICE KURUKANPARA Hrs MV ACT City) VADEKETHALA(H Thrissur 04-01-2020 25, ) 35/2020 U/s East VIMOD, SI BAILED BY 2 Devis santhosh THRISSUR at 10:00 Male CHEROOR,THRISS 151 CrPC (Thrissur OF POLICE POLICE Hrs UR City) Thrissur MUDATHENI (H) 04-01-2020 VENUGOPA 27, 35/2020 U/s East VIMOD, SI BAILED BY 3 ARUN KANJANNI,THRISS THRISSUR at 10:00 L Male 151 CrPC (Thrissur OF POLICE POLICE UR Hrs City) MELEPATTUKIZH GURUVAYO 04-01-2020 GVR Temple JOSHY.G.T.,G SAROJAKSH 47, 11/2020 U/s BAILED BY 4 PRADEEP AKKETHIL HOUSE OR TEMPLE at 15:25 (Thrissur ASI ,TEMPLE AN Male 279, 338 IPC POLICE , OTTAPPALAM . PS Hrs City) P.S. kulangara (h) 03-01-2020 23/2020 U/s Ollur 33, BAILED BY 5 jibin andrews mulayam (po) kuttaneloor at 19:40 13 r/w 63 of (Thrissur s sinoj Male POLICE chavarampadam , Hrs Abkari Act City) POOVAKKULAM HOUSE,VALIYAKU LAM 03-01-2020 10/2020 U/s Peechi 22, DESAM,ANCHUM PEECHI S I BIPIN B BAILED BY 6 JUSTIN JOMON at 19:20 118(a) of KP (Thrissur Male OORTHYMANGAL ROAD JN NAIR POLICE Hrs Act City) AM P.O,VADAKKANC HERY,PALAKKAD CHEMBATH 03-01-2020