Molecular Characterization of Bacterial Communities in the Human Gastrointestinal Tract'
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Methanospirillum Hungatei GP1 As an S Layer MAX FIRTEL,1 GORDON SOUTHAM,1 GEORGE HARAUZ,2 and TERRY J
JOURNAL OF BACTERIOLOGY, Dec. 1993, p. 7550-7560 Vol. 175, No. 23 0021-9193/93/237550-11$02.00/0 Copyright © 1993, American Society for Microbiology Characterization of the Cell Wall of the Sheathed Methanogen Methanospirillum hungatei GP1 as an S Layer MAX FIRTEL,1 GORDON SOUTHAM,1 GEORGE HARAUZ,2 AND TERRY J. BEVERIDGE'* Department ofMicrobiology' and Department ofMolecular Biology and Genetics, 2 College of Biological Sciences, University of Guelph, Guelph, Ontario, Canada N1G 2W1 Received 16 June 1993/Accepted 27 September 1993 The cell wall of MethanospiriUum hungatei GP1 is a labile structure that has been difficult to isolate and Downloaded from characterize because the cells which it encases are contained within a sheath. Cell-sized fragments, 560 nm wide by several micrometers long, of cell wall were extracted by a novel method involving the gradual drying of the filaments in 2% (wtlvol) sodium dodecyl sulfate and 10%0 (wt/vol) sucrose in 50 mM N-2-hydroxyeth- ylpiperazine-N'-2-ethanesulfonic acid (HEPES) buffer containing 10 mM EDTA. The surface was a hexagonal array (a = b = 15.1 nm) possessing a helical superstructure with a ca. 2.50 pitch angle. In shadowed relief, the smooth outer face was punctuated with deep pits, whereas the inner face was relatively featureless. Computer-based two-dimensional reconstructed views of the negatively stained layer demonstrated 4.0- and 2.0-nm-wide electron-dense regions on opposite sides of the layer likely corresponding to the openings of funnel-shaped channels. The face featuring the larger openings best corresponds to the outer face of the layer. -

Characterization of the First Cultured Representative of Verrucomicrobia Subdivision 5 Indicates the Proposal of a Novel Phylum
The ISME Journal (2016) 10, 2801–2816 OPEN © 2016 International Society for Microbial Ecology All rights reserved 1751-7362/16 www.nature.com/ismej ORIGINAL ARTICLE Characterization of the first cultured representative of Verrucomicrobia subdivision 5 indicates the proposal of a novel phylum Stefan Spring1, Boyke Bunk2, Cathrin Spröer3, Peter Schumann3, Manfred Rohde4, Brian J Tindall1 and Hans-Peter Klenk1,5 1Department Microorganisms, Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany; 2Department Microbial Ecology and Diversity Research, Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany; 3Department Central Services, Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany and 4Central Facility for Microscopy, Helmholtz-Centre of Infection Research, Braunschweig, Germany The recently isolated strain L21-Fru-ABT represents moderately halophilic, obligately anaerobic and saccharolytic bacteria that thrive in the suboxic transition zones of hypersaline microbial mats. Phylogenetic analyses based on 16S rRNA genes, RpoB proteins and gene content indicated that strain L21-Fru-ABT represents a novel species and genus affiliated with a distinct phylum-level lineage originally designated Verrucomicrobia subdivision 5. A survey of environmental 16S rRNA gene sequences revealed that members of this newly recognized phylum are wide-spread and ecologically important in various anoxic environments ranging from hypersaline sediments to wastewater and the intestine of animals. Characteristic phenotypic traits of the novel strain included the formation of extracellular polymeric substances, a Gram-negative cell wall containing peptidoglycan and the absence of odd-numbered cellular fatty acids. Unusual metabolic features deduced from analysis of the genome sequence were the production of sucrose as osmoprotectant, an atypical glycolytic pathway lacking pyruvate kinase and the synthesis of isoprenoids via mevalonate. -

Infection Control
infection control JANICE CARR The above photo depicts an E.coli (ATCC 11775) biofilm grown on PC (polycarbonate) coupons using a CDC biofilm reactor. Microorganisms often colonize, and adhere strongly to living and non-living surfaces forming biofilms, and at times, demonstrate an increased resistance to antimicrobials. Biofilms on indwelling medical devices pose a serious threat to public health. BIOFILMS:BIOFILMS: Friend or Foe? By NICOLE KENNY, B.Sc, Assoc.Chem., Director of Professional & Technical Services, Virox Technologies Inc 38 Sanitation Canada - SEPTEMBER / OCTOBER 2006 JANICE CARR Scanning electron micrograph of a Staphylococcus biofilm on the inner surface of a needleless connector. A distinguishing characteristic of biofilms is the presence of extracellular polymeric substances, primarily polysaccharides, surrounding and encasing the cells. Here, there polysaccharides have been visualized by scanning electron microscopy. Picture yourself a con- “Why didn’t I listen to my mother and take Biofilms can be dangerous or benefi- testant on Jeopardy. Alex more science courses?” But in that split cial depending on where they are found Tribec has just asked you second you also remember a documen- and of which organisms they are com- to choose the category. tary you watched on CNN about whirl- prised. In industry, biofilms are responsi- You’re lagging behind the pool tubs and you know the answer. ble for billions of dollars in lost produc- leader by $400. All but “Alex, what are BIOFILMS?” tivity due to equipment damage, notori- one of the $500 questions ously famous for causing pipes to plug or Phave been taken, and the last category has THE ISSUE corrode. -

Actinobacteria and the Vitamin Metabolism of Firebugs
Actinobacteria and the Vitamin Metabolism of Firebugs - Characterizing a mutualism's specificity and functional importance - Seit 1558 Dissertation To Fulfill the Requirements for the Degree of „Doctor of Philosophy“ (PhD) Submitted to the Council of the Faculty of Biology and Pharmacy of the Friedrich Schiller University Jena by M.S. Hassan Salem Born on 16.01.1986 in Cairo, Egypt i Das Promotionsgesuch wurde eingereicht und bewilligt am: Gutachter: 1) 2) 3) Das Promotionskolloquium wurde abgelegt am: ii To Nagla and Samy, for ensuring that life’s possibilities remain endless To Aly, for sharing everything* And to Aileen, my beloved HERC2 mutant * Everything except our first Gameboy (circa 1993). For all else, I am profoundly grateful. i ii CONTENTS LIST OF PUBLICATIONS ................................................................................................... 1 CHAPTER 1: SYMBIOSIS AND THE EVOLUTION OF BIOLOGICAL NOVELTY IN INSECTS ............................................................................................................................ 3 1.1 The organism in the age of the holobiont: It, itself, they .................................................. 3 1.2 Adaptive significance of symbiosis .................................................................................. 4 1.3 Symbiont-mediated diversification ................................................................................... 5 1.4 Revisiting Darwin’s mystery of mysteries: The role of symbiosis in species formation 6 1.5 Homeostasis of symbioses -

Functional Anatomy of Prokaryotes and Eukaryotes
FUNCTIONAL ANATOMY OF PROKARYOTES AND EUKARYOTES BY DR JAWAD NAZIR ASSISTANT PROFESSOR DEPARTMENT OF MICROBIOLOGY UNIVERSITY OF VETERINARY AND ANIMAL SCIENCES, LAHORE Prokaryotes vs Eukaryotes Prokaryote comes from the Greek words for pre-nucleus Eukaryote comes from the Greek words for true nucleus. Functional anatomy of prokaryotes Prokaryotes vs Eukaryotes Prokaryotes Eukaryotes One circular chromosome, not in Paired chromosomes, in nuclear a membrane membrane No histones Histones No organelles Organelles Peptidoglycan cell walls Polysaccharide cell walls Binary fission Mitotic spindle Functional anatomy of prokaryotes Size and shape Average size: 0.2 -1.0 µm 2 - 8 µm Basic shapes: Functional anatomy of prokaryotes Size and shape Pairs: diplococci, diplobacilli Clusters: staphylococci Chains: streptococci, streptobacilli Functional anatomy of prokaryotes Size and shape Functional anatomy of prokaryotes Size and shape Functional anatomy of prokaryotes Size and shape Unusual shapes Star-shaped Stella Square Haloarcula Most bacteria are monomorphic A few are pleomorphic Genus: Stella Genus: Haloarcula Functional anatomy of prokaryotes Bacterial cell structure Structures external to cell wall Cell wall itself Structures internal to cell wall Functional anatomy of prokaryotes Glycocalyx Outside cell wall Usually sticky A capsule is neatly organized A slime layer is unorganized & loose Extracellular polysaccharide allows cell to attach Capsules prevent phagocytosis Association with diseases B. anthracis S. pneumoniae Functional anatomy of prokaryotes Flagella Outside cell wall Filament made of chains of flagellin Attached to a protein hook Anchored to the wall and membrane by the basal body Functional anatomy of prokaryotes Flagella Arrangement Functional anatomy of prokaryotes Bacterial motility Rotate flagella to run or tumble Move toward or away from stimuli (taxis) Flagella proteins are H antigens (e.g., E. -

Microbiome of Odontogenic Abscesses
microorganisms Article Microbiome of Odontogenic Abscesses Sebastian Böttger 1,* , Silke Zechel-Gran 2, Daniel Schmermund 1, Philipp Streckbein 1 , Jan-Falco Wilbrand 1 , Michael Knitschke 1 , Jörn Pons-Kühnemann 3, Torsten Hain 2,4, Markus Weigel 2 , Hans-Peter Howaldt 1, Eugen Domann 4,5 and Sameh Attia 1 1 Department of Oral and Maxillofacial Surgery, Justus-Liebig-University Giessen, University Hospital Giessen and Marburg, Giessen, D-35392 Giessen, Germany; [email protected] (D.S.); [email protected] (P.S.); [email protected] (J.-F.W.); [email protected] (M.K.); [email protected] (H.-P.H.); [email protected] (S.A.) 2 Institute of Medical Microbiology, Justus-Liebig-University Giessen, D-35392 Giessen, Germany; [email protected] (S.Z.-G.); [email protected] (T.H.); [email protected] (M.W.) 3 Institute of Medical Informatics, Justus-Liebig-University Giessen, D-35392 Giessen, Germany; [email protected] 4 German Center for Infection Research (DZIF), Justus-Liebig-University Giessen, Partner Site Giessen-Marburg-Langen, D-35392 Giessen, Germany; [email protected] 5 Institute of Hygiene and Environmental Medicine, Justus-Liebig-University Giessen, D-35392 Giessen, Germany * Correspondence: [email protected]; Tel.: +49-641-98546271 Abstract: Severe odontogenic abscesses are regularly caused by bacteria of the physiological oral Citation: Böttger, S.; Zechel-Gran, S.; microbiome. However, the culture of these bacteria is often prone to errors and sometimes does not Schmermund, D.; Streckbein, P.; result in any bacterial growth. -

Cell Structure and Function in the Bacteria and Archaea
4 Chapter Preview and Key Concepts 4.1 1.1 DiversityThe Beginnings among theof Microbiology Bacteria and Archaea 1.1. •The BacteriaThe are discovery classified of microorganismsinto several Cell Structure wasmajor dependent phyla. on observations made with 2. theThe microscope Archaea are currently classified into two 2. •major phyla.The emergence of experimental 4.2 Cellscience Shapes provided and Arrangements a means to test long held and Function beliefs and resolve controversies 3. Many bacterial cells have a rod, spherical, or 3. MicroInquiryspiral shape and1: Experimentation are organized into and a specific Scientificellular c arrangement. Inquiry in the Bacteria 4.31.2 AnMicroorganisms Overview to Bacterialand Disease and Transmission Archaeal 4.Cell • StructureEarly epidemiology studies suggested how diseases could be spread and 4. Bacterial and archaeal cells are organized at be controlled the cellular and molecular levels. 5. • Resistance to a disease can come and Archaea 4.4 External Cell Structures from exposure to and recovery from a mild 5.form Pili allowof (or cells a very to attach similar) to surfacesdisease or other cells. 1.3 The Classical Golden Age of Microbiology 6. Flagella provide motility. Our planet has always been in the “Age of Bacteria,” ever since the first 6. (1854-1914) 7. A glycocalyx protects against desiccation, fossils—bacteria of course—were entombed in rocks more than 3 billion 7. • The germ theory was based on the attaches cells to surfaces, and helps observations that different microorganisms years ago. On any possible, reasonable criterion, bacteria are—and always pathogens evade the immune system. have been—the dominant forms of life on Earth. -

Novel Molecular, Structural and Evolutionary Characteristics of the Phosphoketolases from Bifidobacteria and Coriobacteriales
RESEARCH ARTICLE Novel molecular, structural and evolutionary characteristics of the phosphoketolases from bifidobacteria and Coriobacteriales Radhey S. Gupta*, Anish Nanda, Bijendra Khadka Department of Biochemistry and Biomedical Sciences, McMaster University, Hamilton, Ontario, Canada * [email protected] a1111111111 a1111111111 a1111111111 Abstract a1111111111 Members from the order Bifidobacteriales, which include many species exhibiting health a1111111111 promoting effects, differ from all other organisms in using a unique pathway for carbohydrate metabolism, known as the ªbifid shuntº, which utilizes the enzyme phosphoketolase (PK) to carry out the phosphorolysis of both fructose-6-phosphate (F6P) and xylulose-5-phosphate (X5P). In contrast to bifidobacteria, the PKs found in other organisms (referred to XPK) are OPEN ACCESS able to metabolize primarily X5P and show very little activity towards F6P. Presently, very lit- Citation: Gupta RS, Nanda A, Khadka B (2017) tle is known about the molecular or biochemical basis of the differences in the two forms of Novel molecular, structural and evolutionary PKs. Comparative analyses of PK sequences from different organisms reported here have characteristics of the phosphoketolases from bifidobacteria and Coriobacteriales. PLoS ONE 12 identified multiple high-specific sequence features in the forms of conserved signature (2): e0172176. doi:10.1371/journal.pone.0172176 inserts and deletions (CSIs) in the PK sequences that clearly distinguish the X5P/F6P phos- Editor: Eugene A. Permyakov, Russian Academy of phoketolases (XFPK) of bifidobacteria from the XPK homologs found in most other organ- Medical Sciences, RUSSIAN FEDERATION isms. Interestingly, most of the molecular signatures that are specific for the XFPK from Received: December 12, 2016 bifidobacteria are also shared by the PK homologs from the Coriobacteriales order of Acti- nobacteria. -
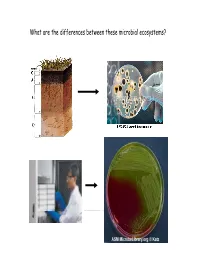
What Are the Differences Between These Microbial Ecosystems? Wwwwe Now Know That, Like Other Org Gm,Anisms, Bacteria Exhibit Social Behaviors
What are the differences between these microbial ecosystems? WWwwe now know that, like other org gm,anisms, bacteria exhibit social behaviors. Bacterial cell escaping for a rare moment of peace and quiet contemplation Sociomicrobiology I. Cell signaling A. Definition/description B. Intraspecific (within spp.): Myxococcus C. Interspecific (between spp.): Pseudomonas aureofaciens II. Biofilms A. Biofilm formation B. Planktonic cells vs. biofilm cells C. General characteristics, structures D. Biofilms as social entities Small diffusible molecules mediate bacterial communication O O N H AHL O O O N H O http://www.ted.com/index.php/talks/bonnie_bassler_on_how_bacteria_communicate.html Why cell-cell signaling in bacteria? Often, single cells in a population might benefit from knowing how many cells are present… “A multitude of bacteria are stronger than a few, thus by union are able overcome obstacles too great for few.” -- Dr. Erwin F. Smith, 1905 (Father of Plant Bacteriology) Pseudomonas aeruginosa in lungs Xylella fastidiosa in xylem Why cell-cell signaling in bacteria? Cell-cell signaling enables bacteria to coordinate behavior to respond quickly to environmental stimuli… such as: -presence of suitable host -change in nutrient availability -defense/competition against other microorganisms -many others! CmmiCommunica tion among btbacteri i:a: The example of Myxococcus xanthus, the Wolf Pack feeder of bacteria http://cmgm.stanford.edu/devbio/kaiserlab/about_myxo/about_myxococcus.html Cooperation among cells in a population: myxobacteria. The myxobacteria are Gram-negative, ubiquitous, soil-dwelling bacteria that are capable of multicellular, social behaviour. In the presence of nutrients, “swarms” of myxobacteria feed cooperatively by sharing extracellular digestive enzymes, and can prey on other bacteria. -

Introduction to Microbiology
Introduction to microbiology Prof. dr hab. Beata M. Sobieszczańska Department of Microbiology University of Medicine • http://www.lekarski.umed.wroc.pl/mikrobiologia • schedules, rules, important information • Consulting hours – teachers are always available for students during consulting hours or classes – apart from consulting hours – you must chase ! • Sick leaves (original) must be shown to the teacher just after an absence but not longer than after two weeks otherwise a sick note will not be honored - a copy of the sick note must be delivered to the secretary office • Class tests – 10 open questions • Terms: 1st, 2nd – if failed commission test from the whole material at the end of semester • Students with the average 4.8 will be released from the final exam • Presence on lectures and classes are obligatory • The final grade from classes is the average of all grades during semester Your best friend in this year: Medical Microbiology by Patrick R. Murray, Ken S. Rosenthal, Michael A. Pfaller Answer questions: • Name important cell wall structures of GP and GN bacteria • What is a role of these structures in human diseases? • Name other than bacterial cell wall structures and explain their role in bacterial pathogenicity • Do you understand the term pathogenicity? • Name five different genera GP and GN bacteria and indicate the colour they have after Gram staining Answer questions: • Name clinically important bacteria producing endospores – why endospores are important? • What is the difference between capsule and glycocalyx layer on GP bacteria? • What is axial filament? What role it plays? What bacteria produce axial filaments? • Name two types of pili and their role in bacterial pathogenicity Most bacteria come in one of three basic shapes: coccus, rod or bacillus & spiral MURRAY 7th ed. -

Trace Elements in Anaerobic Biotechnologies
Trace Elements Biotechnologies in Anaerobic Trace Trace Elements in Anaerobic Biotechnologies Edited by Fernando G. Fermoso, Eric van Hullebusch, Gavin Collins, Jimmy Roussel, Ana Paula Mucha and Giovanni Esposito The use of trace elements to promote biogas production features prominently on the agenda for many biogas-producing companies. However, the application of the technique is often characterized by trial-and-error methodology due to the ambiguous and scarce basic knowledge on the impact of trace elements in anaerobic biotechnologies under different process conditions. This book describes and defines the broad landscape in the research area of trace elements in anaerobic biotechnologies, from the level of advanced chemistry and single microbial cells, through to engineering and bioreactor technology and to the fate of trace elements in the environment. The book results from the EU COST Action on ‘The ecological roles of trace metals in anaerobic biotechnologies’. Trace elements in anaerobic biotechnologies is a critical, exceptionally complex and technical Collins, Gavin Hullebusch, van Eric G. Fermoso, Fernando by Edited challenge. The challenging chemistry underpinning the availability of Esposito and Giovanni Mucha Roussel, Ana Paula Jimmy trace elements for biological uptake is very poorly understood, despite the importance of trace elements for successful anaerobic operations across the bioeconomy. This book discusses and places a common understanding of this challenge, with a strong focus on technological Trace Elements tools and solutions. The group of contributors brings together chemists with engineers, biologists, environmental scientists and mathematical modellers, as well as industry representatives, to show an up-to-date vision of the fate of trace elements on anaerobic biotechnologies. -

Impact of the Interplay Between Bile Acids, Lipids, Intestinal Coriobacteriaceae and Diet on Host Metabolism
Technische Universität München ZIEL – Zentralinstitut für Ernährungs- und Lebensmittelforschung Nachwuchsgruppe Intestinales Mikrobiom Impact of the interplay between bile acids, lipids, intestinal Coriobacteriaceae and diet on host metabolism Sarah Just Vollständiger Abdruck der von der Fakultät Wissenschaftszentrum Weihenstephan für Ernährung, Landnutzung und Umwelt der Technischen Universität München zur Erlangung des akademischen Grades eines Doktors der Naturwissenschaften (Dr. rer. nat.) genehmigten Dissertation. Vorsitzender: Prof. Dr. rer. nat. Siegfried Scherer Prüfer der Dissertation: 1. Prof. Dr. rer. nat. Dirk Haller 2. Prof. Dr. rer. nat. Martin Klingenspor Die Dissertation wurde am 14.02.2017 bei der Technischen Universität München eingereicht und durch die Fakultät Wissenschaftszentrum Weihenstephan für Ernährung, Landnutzung und Umwelt am 12.06.2017 angenommen. Abstract Abstract The gut microbiome is a highly diverse ecosystem which influences host metabolism via for instance via conversion of bile acids and production of short chain fatty acids. Changes in gut microbiota profiles are associated with metabolic diseases such as obesity, type-2 diabetes, and non-alcoholic fatty liver disease. However, beyond alteration of the ecosystem structure, only a handful of specific bacterial species were shown to influence host metabolism and knowledge about molecular mechanisms by which gut bacteria regulate host metabolism are scant. The family Coriobacteriaceae (phylum Actinobacteria) comprises dominant members of the human gut microbiome and can metabolize cholesterol-derived substrates such as bile acids. Furthermore, their occurrence has been associated with alterations of lipid and cholesterol metabolism. However, consequences for the host are unknown. Hence, the aim of the present study was to characterize the impact of Coriobacteriaceae on lipid, cholesterol, and bile acid metabolism in vivo.