WO 2012/107206 Al 16 August 2012 (16.08.2012) P O P C T
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

WO 2016/023103 Al 18 February 2016 (18.02.2016) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization I International Bureau (10) International Publication Number (43) International Publication Date WO 2016/023103 Al 18 February 2016 (18.02.2016) P O P C T (51) International Patent Classification: (74) Agent: BEN-OLIEL, Susan Margaret; Fasken Martineau C07H 15/256 (2006.01) A61K 36/28 (2006.01) DuMoulin LLP, 2900-550 Burrard Street, Vancouver, BC A23L 1/236 (2006.01) C07H 15/24 (2006.01) V6C 0A3 (CA). A23L 2/60 (2006.01) (81) Designated States (unless otherwise indicated, for every (21) International Application Number: kind of national protection available): AE, AG, AL, AM, PCT/CA20 15/000462 AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, (22) International Filing Date: DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, 12 August 2015 (12.08.2015) HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, (25) Filing Language: English KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (26) Publication Language: English PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, (30) Priority Data: SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, 2014 10393477.0 12 August 2014 (12.08.2014) CN TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (71) Applicant: LI, Cunbiao Kevin [CA/CA]; c/o GLG Life (84) Designated States (unless otherwise indicated, for every Tech Corporation, 2168-1050 West Pender Street, Van kind of regional protection available): ARIPO (BW, GH, couver, British Columbia V6E 3S7 (CA). -

WO 2015/183714 Al 3 December 2015 (03.12.2015) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2015/183714 Al 3 December 2015 (03.12.2015) P O P C T (51) International Patent Classification: Wilmington, Delaware 19805 (US). ROTHMAN, Steven C12P 19/08 (2006.01) A23L 1/054 (2006.01) Cary; 101 Lassen Court Apt 7, Princeton, New Jersey C12P 19/04 (2006.01) A61K 31/716 (2006.01) 08540 (US). CUP 19/18 (2006.01) A61K 8/36 (2006.01) (81) Designated States (unless otherwise indicated, for every C08B 37/00 (2006.01) kind of national protection available): AE, AG, AL, AM, (21) International Application Number: AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, PCT/US20 15/032 106 BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 22 May 2015 (22.05.2015) KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, (25) Filing Language: English MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, (26) Publication Language: English SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, (30) Priority Data: TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. 62/004,305 29 May 2014 (29.05.2014) US (84) Designated States (unless otherwise indicated, for every (71) Applicant: E. -

High-Purity Rebaudioside D and Applications Hochreines Rebaudiosid-D Und Anwendungen Rébaudioside D De Grande Pureté Et Applications
(19) TZZ Z_T (11) EP 2 708 548 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C07H 1/08 (2006.01) A21D 2/36 (2006.01) 06.12.2017 Bulletin 2017/49 A23G 1/42 (2006.01) (21) Application number: 13196410.8 (22) Date of filing: 13.10.2010 (54) High-Purity Rebaudioside D and Applications Hochreines Rebaudiosid-D und Anwendungen Rébaudioside D de grande pureté et applications (84) Designated Contracting States: (72) Inventors: AL AT BE BG CH CY CZ DE DK EE ES FI FR GB • Abelyan, Varuzhan GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO 50480 Kuala Lumpur (MY) PL PT RO RS SE SI SK SM TR • Markosyan, Avetik 59200 Kuala Lumpur (MY) (30) Priority: 15.10.2009 US 580233 • Abelyan, Lidia 24.05.2010 US 785501 50480 Kuala Lumpur (MY) 24.05.2010 US 785504 24.05.2010 US 785506 (74) Representative: Hocking, Adrian Niall et al 24.05.2010 US 785507 Albright IP Limited 24.05.2010 US 785508 County House 24.05.2010 US 786392 Bayshill Road 24.05.2010 US 786402 Cheltenham, Glos. GL50 3BA (GB) 24.05.2010 US 786413 24.05.2010 US 786416 (56) References cited: 24.05.2010 US 786427 WO-A1-2009/071277 24.05.2010 US 786430 24.05.2010 US 786419 • I. SAKAMOTO ET AL: "Application of 13C NMR spectroscopy to chemistry of natural glycosices: (43) Date of publication of application: rebaudioside-C,a newsweet diterpeneglycoside 19.03.2014 Bulletin 2014/12 of Stevia rebaudiana", CHEM. -
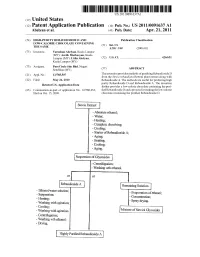
Stevia Extract I
US 20110091637A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0091637 A1 Abelyan et al. (43) Pub. Date: Apr. 21, 2011 (54) HIGH-PURITY REBAUDIOSIDE D AND Publication Classi?cation LOW-CALORIE CHOCOLATE CONTAINING THE SAME (51) Int. Cl. A23G 1/40 (2006.01) (75) Inventors: Varuzhan Abelyan, Kuala Lumpur (MY); Avetik Markosyan, Kuala Lumpur (MY); Lidia Abelyan, (52) US. Cl. ...................................................... .. 426/631 Kuala Lumpur (MY) (73) Assignee: PureCircle Sdn Bhd, Negeri Semb?an (MY) (57) ABSTRACT 21 A 1. N .._ 12/785 507 The invention P rovides methods of P urifyin g RebaudiosideD ( ) pp 0 ’ from the Slevia rebaudiana Bertoni plant extract along With (22) Filed: May 24, 2010 Rebaudioside A. The methods are useful for producing high purity Rebaudioside D and Rebaudioside A. The invention Related US. Application Data further provides a loW-calorie chocolate containing the puri (63) Continuation-in-part of application No, 12/580,233, ?ed Rebaudioside D and a process for making the low-calorie ?led on Oct 15, 2009 chocolate containing the puri?ed Rebaudioside D. Stevia Extract I - Absolute ethanol; - Water; ~ Heating; - Complete dissolving; - Cooling; - Starter of Rebaudioside A; - Aging; - Heating; - Cooling; - Aging. Suspension of Glycosides - centri?lgation; - Washing wth ethanol. or or R?baudloslde A Remaining Solution - Ethanoi/ t ' ' ' ' _ suspensi‘gz. e1 Soiuilon, - Evaporation of ethanol; “ Heating. , - Concentration; - Washmg- _ a with_ agitation;. I ~ Spray drying. - Cooling; . “ Washing with agitation. Mixture of Stevio] Glycosides » Cennifugation; - Washing wth ethanol; ~ Drying. Highly Puri?ed Rebaudioside A Patent Application Publication Apr. 21, 2011 Sheet 2 0f 11 US 2011/0091637 A1 Stevioside FIGZ Patent Application Publication Apr. -

Alternative-Sweeteners-2001.Pdf
ISBN: 0-8247-0437-1 This book is printed on acid-free paper. Headquarters Marcel Dekker, Inc. 270 Madison Avenue, New York, NY 10016 tel: 212-696-9000; fax: 212-685-4540 Eastern Hemisphere Distribution Marcel Dekker AG Hutgasse 4, Postfach 812, CH-4001 Basel, Switzerland tel: 41-61-261-8482; fax: 41-61-261-8896 World Wide Web http://www.dekker.com The publisher offers discounts on this book when ordered in bulk quantities. For more information, write to Special Sales/Professional Marketing at the headquarters address above. Copyright 2001 by Marcel Dekker, Inc. All Rights Reserved. Neither this book nor any part may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, microfilming, and recording, or by any information storage and retrieval system, without permission in writing from the publisher. Current printing (last digit): 10987654321 PRINTED IN THE UNITED STATES OF AMERICA Preface Alternative sweeteners, both as a group and in some cases individually, are among the most studied food ingredients. Controversy surrounding them dates back al- most a century. Consumers are probably more aware of sweeteners than any other category of food additive. The industry continues to develop new sweeteners, each declared better than the alternatives preceding it and duplicative of the taste of sugar, the gold standard for alternative sweeteners. In truth, no sweetener is perfect—not even sugar. Combination use is often the best alternative. While new developments in alternative sweeteners continue to abound, their history remains fascinating. Saccharin and cyclamates, among the earliest of the low-calorie sweeteners, have served as scientific test cases. -

Natural Sweeteners : a Complete Review Keerthi Priya*, Dr.Vankadari Rama Mohan Gupta, Kalakoti Srikanth Daewoong Pharmaceutical Co
Keerthi Priya et al. / Journal of Pharmacy Research 2011,4(7),2034-2039 Review Article Available online through ISSN: 0974-6943 http://jprsolutions.info Natural Sweeteners : A Complete Review Keerthi Priya*, Dr.Vankadari Rama Mohan Gupta, Kalakoti Srikanth Daewoong Pharmaceutical Co. Ltd. Balanagar,Hyderabad-500 037, Andhraprdesh, India Received on: 12-04-2011; Revised on: 18-05-2011; Accepted on:21-06-2011 ABSTRACT Two types of sweeteners are available: natural sweeteners of plant origin and artificial or synthetic sweeteners. Sweetening agents either evoke sweet taste or enhance the perception of sweet taste. Natural sweetening agents are preferred over synthetic sweetening agents since they do not have any adverse impact on health. Non-saccharide natural sweetening agents are low calorific, nontoxic and super sweet (100 to 10,000 times sweeter than sugar) in nature and can overcome the problems of sucrose and synthetic sweeteners. Natural sweeteners are useful sugar substitutes for diabetic patients. The active sweet principles stored in plants can be grouped under: terpenoids, steroidal saponins, dihydroisocoumarins, dihydrochalcones, proteins, polyols, volatile oils, etc. in nature. Common and scientific names of these sweeteners along with their properties, chemical structure of sweet principles, pharmaceutical uses have been presented in this paper. Key words: Natural sweetening agents, Saccharide sweeteners, Non-saccharide sweeteners. Terpenoids, Polyols INTRODUCTION Preference for sweet taste at a range of intensities is characteristic of human 3.Sugar is also employed in the coating of pills and tablets species. In the fetus, taste buds are developed by the 16th week of gestation, and 4.Honey plays an important role in Ayurvedic system of medicine. -

Wo 2009/108680 A2
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date 3 September 2009 (03.09.2009) WO 2009/108680 A2 (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C07H 15/256 (2006.01) A23L 1/236 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, (21) International Application Number: CA, CH, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, PCT/US2009/035 108 EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, (22) International Filing Date: HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, KR, 25 February 2009 (25.02.2009) KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, (25) Filing Language: English NZ, OM, PG, PH, PL, PT, RO, RS, RU, SC, SD, SE, SG, (26) Publication Language: English SK, SL, SM, ST, SV, SY, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: 61/03 1,300 25 February 2008 (25.02.2008) US (84) Designated States (unless otherwise indicated, for every 61/140,646 24 December 2008 (24.12.2008) US kind of regional protection available): ARIPO (BW, GH, GM, KE, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, ZM, (71) Applicant (for all designated States except US): THE ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, COCA-COLA COMPANY [US/US]; One Coca-cola TM), European (AT, BE, BG, CH, CY, CZ, DE, DK, EE, Plaza, NW, Atlanta, GA 303 13 (US).