Alternative-Sweeteners-2001.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) Patent Application Publication (10) Pub. No.: US 2017/0143022 A1 Wicker Et Al
US 20170143022A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0143022 A1 Wicker et al. (43) Pub. Date: May 25, 2017 (54) COMPOSITIONS INCORPORATING AN (52) U.S. Cl. UMLAM FLAVORAGENT CPC ............... A2.3L 27/20 (2016.08); A23L 27/88 (2016.08); A23L 2/56 (2013.01); A23L 2780 (71) Applicant: Senomyx, Inc., San Diego, CA (US) (2016.08); A23L 27/30 (2016.08); A23K 20/10 (2016.05); A23K 50/40 (2016.05); A61K 47/22 (72) Inventors: Sharon Wicker, Carlsbad, CA (US); (2013.01) Tanya Ditschun, San Diego, CA (US) (21) Appl. No.: 14/948,101 (57) ABSTRACT The present invention relates to compositions containing (22) Filed: Nov. 20, 2015 flavor or taste modifiers, such as a flavoring or flavoring agents and flavor or taste enhancers, more particularly, Publication Classification savory (“umami”) taste modifiers, savory flavoring agents (51) Int. Cl. and savory flavor enhancers, for foods, beverages, and other AOIN 25/00 (2006.01) comestible compositions. Compositions comprising an A23K2O/It (2006.01) umami flavor agent or umami taste-enhancing agent in A6 IK 47/22 (2006.01) combination with one or more other food additives, prefer A2.3L 2/56 (2006.01) ably including a flavorant, herb, Spice, fat, or oil, are A23K 50/40 (2006.01) disclosed. US 2017/O 143022 A1 May 25, 2017 COMPOSITIONS INCORPORATING AN tions WO 02/06254, WO 00/63166 art, WO 02/064631, and UMAM FLAVORAGENT WO 03/001876, and U.S. Patent publication US 2003 0232407 A1. The entire disclosures of the articles, patent BACKGROUND OF THE INVENTION applications, -

Food Additives and Ingredients Course Teacher
STUDY MATERIAL Class : M. Sc. Previous Year IInd Semester Department of Food Science & Technology Course Name : Food Additives and Ingredients Course Teacher : Dr. Smt. Alpana Singh A) Theory Lecture Outlines 1. Introduction: What are Food Additives? - Role of Food Additives in Food Processing -functions - Classification - Intentional & Unintentional Food Additives 2. Toxicology and Safety Evaluation of Food Additives - Beneficial effects of Food Additives /Toxic Effects - Food Additives generally recognized as safe (GRAS) - Tolerance levels &Toxic levels in Foods - LD 50 Values of Food additives. 3. Naturally occurring Food Additives - Classification - Role in Food Processing – Health Implications. 4. Food colors - What are food colors - Natural Food Colors - Synthetic food colors - types -their chemical nature - their impact on health. 5. Preservatives - What are preservatives - natural preservation- chemical preservatives – their chemical action on foods and human system. 6. Anti-oxidants & chelating agents - what are anti oxidants - their role in foods - types of antioxidants - natural & synthetic - examples - what are chelating agents - their mode of action in foods - examples. 7. Surface active agents - What are surface active agents - their mode of action in foods -examples. 8. Stabilizers & thickeners - examples - their role in food processing. 9. Bleaching & maturing agents: what is bleaching - Examples of bleaching agents - What is maturing - examples of maturing agents - their role in food processing. 10. Starch modifiers: what are starch modifiers - chemical nature - their role in food processing. 11. Buffers - Acids & Alkalis - examples - types - their role in food processing. 12. Sweeteners - what are artificial sweeteners & non nutritive sweeteners - special dietary supplements & their health implication - role in food processing. 13. Flavoring agents - natural flavors & synthetic flavors - examples & their chemical nature -role of flavoring agents in food processing. -

Can “Functional Sweetener” Context Increase Liking for Cookies Formulated with Alternative Sweeteners?
foods Article Can “Functional Sweetener” Context Increase Liking for Cookies Formulated with Alternative Sweeteners? Soo-Hyun Lee 1 , Seo-Youn Choe 2, Ga-Gyeong Seo 3 and Jae-Hee Hong 1,3,* 1 Research Institute of Human Ecology, Seoul University, Seoul 03080, Korea; [email protected] 2 Department of Food and Nutrition, College of Science and Technology, Kookmin University, Seoul 02707, Korea; [email protected] 3 Department of Food and Nutrition, College of Human Ecology, Seoul University, Seoul 03080, Korea; [email protected] * Correspondence: [email protected]; Tel.: +82-2-880-6837 Abstract: Various strategies for replacing sugar with naturally derived sweeteners are being devel- oped and tested. In this study, the effect of the “functional sweetener” context, which is created by providing health-promoting information, on liking for the sweeteners was investigated using a cookie model system. Cookie samples were prepared by replacing the sugar of 100% sucrose cookies (control) with phyllodulcin, rebaudioside A, xylobiose and sucralose either entirely or partly. The sen- sory profile of the samples was obtained using descriptive evaluations. Hedonic responses to cookie samples were collected from 96 consumers under blind and informed conditions. Replacement of 100% sucrose with rebaudioside A or phyllodulcin significantly increased bitterness but replacement of 50% sugar elicited sensory characteristics similar to those of the control. Although the “functional sweetener” context did not influence overall liking, liking for the samples was more clearly distin- guished when information was provided. Consumers were segmented into three clusters according to their shift in liking in the informed condition: when information was presented, some consumers Citation: Lee, S.-H.; Choe, S.-Y.; Seo, decreased their liking for sucralose cookies, while other consumers increased or decreased their G.-G.; Hong, J.-H. -

WO 2016/023103 Al 18 February 2016 (18.02.2016) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization I International Bureau (10) International Publication Number (43) International Publication Date WO 2016/023103 Al 18 February 2016 (18.02.2016) P O P C T (51) International Patent Classification: (74) Agent: BEN-OLIEL, Susan Margaret; Fasken Martineau C07H 15/256 (2006.01) A61K 36/28 (2006.01) DuMoulin LLP, 2900-550 Burrard Street, Vancouver, BC A23L 1/236 (2006.01) C07H 15/24 (2006.01) V6C 0A3 (CA). A23L 2/60 (2006.01) (81) Designated States (unless otherwise indicated, for every (21) International Application Number: kind of national protection available): AE, AG, AL, AM, PCT/CA20 15/000462 AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, (22) International Filing Date: DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, 12 August 2015 (12.08.2015) HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, (25) Filing Language: English KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (26) Publication Language: English PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, (30) Priority Data: SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, 2014 10393477.0 12 August 2014 (12.08.2014) CN TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (71) Applicant: LI, Cunbiao Kevin [CA/CA]; c/o GLG Life (84) Designated States (unless otherwise indicated, for every Tech Corporation, 2168-1050 West Pender Street, Van kind of regional protection available): ARIPO (BW, GH, couver, British Columbia V6E 3S7 (CA). -

Carbohydrates: Occurrence, Structures and Chemistry
Carbohydrates: Occurrence, Structures and Chemistry FRIEDER W. LICHTENTHALER, Clemens-Schopf-Institut€ fur€ Organische Chemie und Biochemie, Technische Universit€at Darmstadt, Darmstadt, Germany 1. Introduction..................... 1 6.3. Isomerization .................. 17 2. Monosaccharides ................. 2 6.4. Decomposition ................. 18 2.1. Structure and Configuration ...... 2 7. Reactions at the Carbonyl Group . 18 2.2. Ring Forms of Sugars: Cyclic 7.1. Glycosides .................... 18 Hemiacetals ................... 3 7.2. Thioacetals and Thioglycosides .... 19 2.3. Conformation of Pyranoses and 7.3. Glycosylamines, Hydrazones, and Furanoses..................... 4 Osazones ..................... 19 2.4. Structural Variations of 7.4. Chain Extension................ 20 Monosaccharides ............... 6 7.5. Chain Degradation. ........... 21 3. Oligosaccharides ................. 7 7.6. Reductions to Alditols ........... 21 3.1. Common Disaccharides .......... 7 7.7. Oxidation .................... 23 3.2. Cyclodextrins .................. 10 8. Reactions at the Hydroxyl Groups. 23 4. Polysaccharides ................. 11 8.1. Ethers ....................... 23 5. Nomenclature .................. 15 8.2. Esters of Inorganic Acids......... 24 6. General Reactions . ............ 16 8.3. Esters of Organic Acids .......... 25 6.1. Hydrolysis .................... 16 8.4. Acylated Glycosyl Halides ........ 25 6.2. Dehydration ................... 16 8.5. Acetals ....................... 26 1. Introduction replacement of one or more hydroxyl group (s) by a hydrogen atom, an amino group, a thiol Terrestrial biomass constitutes a multifaceted group, or similar heteroatomic groups. A simi- conglomeration of low and high molecular mass larly broad meaning applies to the word ‘sugar’, products, exemplified by sugars, hydroxy and which is often used as a synonym for amino acids, lipids, and biopolymers such as ‘monosaccharide’, but may also be applied to cellulose, hemicelluloses, chitin, starch, lignin simple compounds containing more than one and proteins. -

(12) United States Patent (10) Patent No.: US 7,833,555 B2 Andersen Et Al
US007833555B2 (12) United States Patent (10) Patent No.: US 7,833,555 B2 Andersen et al. (45) Date of Patent: *Nov. 16, 2010 (54) CHEWING GUM COMPRISING AT LEAST 2001/0002998 A1 6/2001 Ream et al. TWO DIFFERENT BODEGRADABLE 2004/O115305 A1 6/2004 Andersen et al. POLYMERS 2004/O142066 A1 7/2004 Andersen et al. 2004/O146599 A1 7/2004 Andersen et al. (75) Inventors: Lone Andersen, Middelfart (DK); Helle 2004/O156949 A1 8/2004 Andersen et al. Wittorff, Vejle Ost (DK) 2004/O1801 11 A1 9/2004 Andersen et al. 2006/005.1455 A1 3/2006 Andersen et al. (73) Assignee: Gumlink A/S (DK) 2006.0099300 A1 5/2006 Andersen et al. 2006/0121156 A1 6/2006 Andersen et al. (*) Notice: Subject to any disclaimer, the term of this 2006, O147580 A1 7/2006 Nissen et al. patent is extended or adjusted under 35 U.S.C. 154(b) by 728 days. FOREIGN PATENT DOCUMENTS This patent is Subject to a terminal dis EP O 151344 8, 1985 claimer. EP O 258 780 3, 1988 EP O 415 656 3, 1991 (21) Appl. No.: 11/088,109 EP O427.185 5, 1991 EP O 500 098 8, 1992 (22) Filed: Mar. 23, 2005 EP O 558965 9, 1993 EP 1066 759 1, 2001 (65) Prior Publication Data EP 0 711 506 4/2003 EP 1306 013 5, 2003 US 2005/0244538A1 Nov. 3, 2005 EP 1354908 10, 2003 JP 08-196214 8, 1996 Related U.S. Application Data JP O9-047226 2, 1997 (63) Continuation of application No. -

WO 2015/183714 Al 3 December 2015 (03.12.2015) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2015/183714 Al 3 December 2015 (03.12.2015) P O P C T (51) International Patent Classification: Wilmington, Delaware 19805 (US). ROTHMAN, Steven C12P 19/08 (2006.01) A23L 1/054 (2006.01) Cary; 101 Lassen Court Apt 7, Princeton, New Jersey C12P 19/04 (2006.01) A61K 31/716 (2006.01) 08540 (US). CUP 19/18 (2006.01) A61K 8/36 (2006.01) (81) Designated States (unless otherwise indicated, for every C08B 37/00 (2006.01) kind of national protection available): AE, AG, AL, AM, (21) International Application Number: AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, PCT/US20 15/032 106 BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 22 May 2015 (22.05.2015) KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, (25) Filing Language: English MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, (26) Publication Language: English SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, (30) Priority Data: TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. 62/004,305 29 May 2014 (29.05.2014) US (84) Designated States (unless otherwise indicated, for every (71) Applicant: E. -

High-Purity Rebaudioside D and Applications Hochreines Rebaudiosid-D Und Anwendungen Rébaudioside D De Grande Pureté Et Applications
(19) TZZ Z_T (11) EP 2 708 548 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C07H 1/08 (2006.01) A21D 2/36 (2006.01) 06.12.2017 Bulletin 2017/49 A23G 1/42 (2006.01) (21) Application number: 13196410.8 (22) Date of filing: 13.10.2010 (54) High-Purity Rebaudioside D and Applications Hochreines Rebaudiosid-D und Anwendungen Rébaudioside D de grande pureté et applications (84) Designated Contracting States: (72) Inventors: AL AT BE BG CH CY CZ DE DK EE ES FI FR GB • Abelyan, Varuzhan GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO 50480 Kuala Lumpur (MY) PL PT RO RS SE SI SK SM TR • Markosyan, Avetik 59200 Kuala Lumpur (MY) (30) Priority: 15.10.2009 US 580233 • Abelyan, Lidia 24.05.2010 US 785501 50480 Kuala Lumpur (MY) 24.05.2010 US 785504 24.05.2010 US 785506 (74) Representative: Hocking, Adrian Niall et al 24.05.2010 US 785507 Albright IP Limited 24.05.2010 US 785508 County House 24.05.2010 US 786392 Bayshill Road 24.05.2010 US 786402 Cheltenham, Glos. GL50 3BA (GB) 24.05.2010 US 786413 24.05.2010 US 786416 (56) References cited: 24.05.2010 US 786427 WO-A1-2009/071277 24.05.2010 US 786430 24.05.2010 US 786419 • I. SAKAMOTO ET AL: "Application of 13C NMR spectroscopy to chemistry of natural glycosices: (43) Date of publication of application: rebaudioside-C,a newsweet diterpeneglycoside 19.03.2014 Bulletin 2014/12 of Stevia rebaudiana", CHEM. -
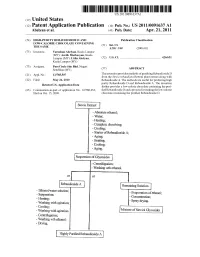
Stevia Extract I
US 20110091637A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0091637 A1 Abelyan et al. (43) Pub. Date: Apr. 21, 2011 (54) HIGH-PURITY REBAUDIOSIDE D AND Publication Classi?cation LOW-CALORIE CHOCOLATE CONTAINING THE SAME (51) Int. Cl. A23G 1/40 (2006.01) (75) Inventors: Varuzhan Abelyan, Kuala Lumpur (MY); Avetik Markosyan, Kuala Lumpur (MY); Lidia Abelyan, (52) US. Cl. ...................................................... .. 426/631 Kuala Lumpur (MY) (73) Assignee: PureCircle Sdn Bhd, Negeri Semb?an (MY) (57) ABSTRACT 21 A 1. N .._ 12/785 507 The invention P rovides methods of P urifyin g RebaudiosideD ( ) pp 0 ’ from the Slevia rebaudiana Bertoni plant extract along With (22) Filed: May 24, 2010 Rebaudioside A. The methods are useful for producing high purity Rebaudioside D and Rebaudioside A. The invention Related US. Application Data further provides a loW-calorie chocolate containing the puri (63) Continuation-in-part of application No, 12/580,233, ?ed Rebaudioside D and a process for making the low-calorie ?led on Oct 15, 2009 chocolate containing the puri?ed Rebaudioside D. Stevia Extract I - Absolute ethanol; - Water; ~ Heating; - Complete dissolving; - Cooling; - Starter of Rebaudioside A; - Aging; - Heating; - Cooling; - Aging. Suspension of Glycosides - centri?lgation; - Washing wth ethanol. or or R?baudloslde A Remaining Solution - Ethanoi/ t ' ' ' ' _ suspensi‘gz. e1 Soiuilon, - Evaporation of ethanol; “ Heating. , - Concentration; - Washmg- _ a with_ agitation;. I ~ Spray drying. - Cooling; . “ Washing with agitation. Mixture of Stevio] Glycosides » Cennifugation; - Washing wth ethanol; ~ Drying. Highly Puri?ed Rebaudioside A Patent Application Publication Apr. 21, 2011 Sheet 2 0f 11 US 2011/0091637 A1 Stevioside FIGZ Patent Application Publication Apr. -

(12) Patent Application Publication (10) Pub. No.: US 2003/0096047 A1 Riha, III Et Al
US 20030096.047A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2003/0096047 A1 Riha, III et al. (43) Pub. Date: May 22, 2003 (54) LOW CALORIE BEVERAGES CONTAINING (60) Provisional application No. 60/179,833, filed on Feb. HIGH INTENSITY SWEETENERS AND 2, 2000. ARABINOGALACTAN Publication Classification (76) Inventors: William E. Riha III, Somerset, NJ (US); Martin Jager, Gauersheim (DE) (51) Int. Cl. ................................................. A23L 11236 (52) U.S. Cl. .............................................................. 426/548 Correspondence Address: Ferrells, PLLC P.O. BOX 312 (57) ABSTRACT Clifton, VA 20124-1706 (US) (21) Appl. No.: 10/245,129 Low calorie beverages Sweetened with high intensity Sweet eners are provided with arabinogalactan in an amount effec (22) Filed: Sep. 17, 2002 tive to mask bitter aftertaste or other off-note sensory Related U.S. Application Data characteristics associated with the high intensity Sweeteners. Particularly preferred embodiments include blends of high (63) Continuation of application No. 09/761,302, filed on intensity SweetenerS and ratioS of arabinogalactan to each Jan. 17, 2001, now abandoned. high intensity Sweetener in the blend of at least about 40. US 2003/0096047 A1 May 22, 2003 LOW CALORIE BEVERAGES CONTAINING HIGH products. Also included are anti-flatulent agents used to help INTENSITY SWEETENERS AND break up the gas created as the polysaccharides are metabo ARABINOGALACTAN lized by the intestinal microflora. 0008 WIPO Publication No. WO 99/17618 (Wrigley) CLAIM FOR PRIORITY discloses chewing gums containing arabinogalactan and 0001. This non-provisional application claims the benefit methods of making Such gums. In one embodiment the gum of the filing date of U.S. -

Risk Assessment Report Advantame (Food Additives)
[Tentative translation] Risk Assessment Report Advantame (Food Additives) Food Safety Commission of Japan (FSCJ) July 2013 1 [Tentative translation] Contents Page Chronology of Discussions ................................................................................................. 3 List of members of the Food Safety Commission of Japan (FSCJ) ................................. 3 List of members of the Expert Committee on Food Additives, the Food Safety Commission of Japan (FSCJ)............................................................................................. 4 Executive summary............................................................................................................. 5 I. Outline of the items under assessment ........................................................................... 6 1. Use ................................................................................................................................ 6 2. Names of the principal components ........................................................................... 6 3. Molecular and structural formulae ........................................................................... 6 4. Molecular weights ....................................................................................................... 6 5. Characteristics ............................................................................................................ 6 6. Stability ....................................................................................................................... -

WO 2016/154503 Al 29 September 2016 (29.09.2016) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/154503 Al 29 September 2016 (29.09.2016) P O P C T (51) International Patent Classification: Saujanya; 33 12 Cornelia Court, Jamestown, NC 27282 A61K 9/48 (2006.01) A61K 47/12 (2006.01) (US). YANG, Chue; 302 Kensington Road, Greensboro, A61K 9/64 (2006.01) A61K 47/42 (2006.01) NC 27403 (US). VAN DUIJNHOVEN, Henricus A61K 9/66 (2006.01) Marinus, Gerardus, Maria; Loevestein 9, 5235 GC Den Bosch (NL). PIEST, Martin; Smijersstraat 66, 5012 CD (21) International Application Number: Tilburg (NL). FATMI, Aqeel, A.; 4125 Premier Drive, PCT/US20 16/024 127 High Point, NC 27265 (US). (22) International Filing Date: (74) Agent: BROWN, Bernard, A., II; Brinks Gilson & Lione, 25 March 2016 (25.03.2016) Post Office Box 110285, Research Triangle Park, NC (25) Filing Language: English 27709 (US). (26) Publication Language: English (81) Designated States (unless otherwise indicated, for every kind of national protection available): AE, AG, AL, AM, (30) Priority Data: AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, 62/138,468 26 March 2015 (26.03.2015) US BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, 62/236,297 2 October 201 5 (02. 10.2015) US DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (71) Applicant: BANNER LIFE SCIENCES LLC [US/US]; HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 4125 Premier Drive, High Point, NC 27265 (US).