Tuberculosis Prevalence Survey
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Districts of Ethiopia
Region District or Woredas Zone Remarks Afar Region Argobba Special Woreda -- Independent district/woredas Afar Region Afambo Zone 1 (Awsi Rasu) Afar Region Asayita Zone 1 (Awsi Rasu) Afar Region Chifra Zone 1 (Awsi Rasu) Afar Region Dubti Zone 1 (Awsi Rasu) Afar Region Elidar Zone 1 (Awsi Rasu) Afar Region Kori Zone 1 (Awsi Rasu) Afar Region Mille Zone 1 (Awsi Rasu) Afar Region Abala Zone 2 (Kilbet Rasu) Afar Region Afdera Zone 2 (Kilbet Rasu) Afar Region Berhale Zone 2 (Kilbet Rasu) Afar Region Dallol Zone 2 (Kilbet Rasu) Afar Region Erebti Zone 2 (Kilbet Rasu) Afar Region Koneba Zone 2 (Kilbet Rasu) Afar Region Megale Zone 2 (Kilbet Rasu) Afar Region Amibara Zone 3 (Gabi Rasu) Afar Region Awash Fentale Zone 3 (Gabi Rasu) Afar Region Bure Mudaytu Zone 3 (Gabi Rasu) Afar Region Dulecha Zone 3 (Gabi Rasu) Afar Region Gewane Zone 3 (Gabi Rasu) Afar Region Aura Zone 4 (Fantena Rasu) Afar Region Ewa Zone 4 (Fantena Rasu) Afar Region Gulina Zone 4 (Fantena Rasu) Afar Region Teru Zone 4 (Fantena Rasu) Afar Region Yalo Zone 4 (Fantena Rasu) Afar Region Dalifage (formerly known as Artuma) Zone 5 (Hari Rasu) Afar Region Dewe Zone 5 (Hari Rasu) Afar Region Hadele Ele (formerly known as Fursi) Zone 5 (Hari Rasu) Afar Region Simurobi Gele'alo Zone 5 (Hari Rasu) Afar Region Telalak Zone 5 (Hari Rasu) Amhara Region Achefer -- Defunct district/woredas Amhara Region Angolalla Terana Asagirt -- Defunct district/woredas Amhara Region Artuma Fursina Jile -- Defunct district/woredas Amhara Region Banja -- Defunct district/woredas Amhara Region Belessa -- -

(ENGINE), Livelihood & Economic Strengthening S
Empowering New Generations to Improve Nutrition and Economic opportunities (ENGINE), Livelihood & Economic Strengthening Sub-Component Assessment of the performance of FTCs and School Gardens supported by ENGINE to undertake Demonstrations (agronomic &cooking practices) in the previous two years of the project (2011/12 & 2012/13) March 2014 Addis Ababa 0 Contents Acronyms ...................................................................................................................................................... 2 Introduction .................................................................................................................................................. 5 Objectives of the assessment ....................................................................................................................... 5 Method of data collection and analysis ......................................................................................................... 6 Findings of the Assessment ........................................................................................................................... 6 1. Farmers Training Centres (FTCs)......................................................................................................... 6 Summary and Recommendations (FTCs) ................................................................................................... 15 I. FTCs with no access to water for irrigation, unfavorable soil and slope of land for vegetable production and no demonstration conducted -

Ethiopia Round 6 SDP Questionnaire
Ethiopia Round 6 SDP Questionnaire Always 001a. Your name: [NAME] Is this your name? ◯ Yes ◯ No 001b. Enter your name below. 001a = 0 Please record your name 002a = 0 Day: 002b. Record the correct date and time. Month: Year: ◯ TIGRAY ◯ AFAR ◯ AMHARA ◯ OROMIYA ◯ SOMALIE BENISHANGUL GUMZ 003a. Region ◯ ◯ S.N.N.P ◯ GAMBELA ◯ HARARI ◯ ADDIS ABABA ◯ DIRE DAWA filter_list=${this_country} ◯ NORTH WEST TIGRAY ◯ CENTRAL TIGRAY ◯ EASTERN TIGRAY ◯ SOUTHERN TIGRAY ◯ WESTERN TIGRAY ◯ MEKELE TOWN SPECIAL ◯ ZONE 1 ◯ ZONE 2 ◯ ZONE 3 ZONE 5 003b. Zone ◯ ◯ NORTH GONDAR ◯ SOUTH GONDAR ◯ NORTH WELLO ◯ SOUTH WELLO ◯ NORTH SHEWA ◯ EAST GOJAM ◯ WEST GOJAM ◯ WAG HIMRA ◯ AWI ◯ OROMIYA 1 ◯ BAHIR DAR SPECIAL ◯ WEST WELLEGA ◯ EAST WELLEGA ◯ ILU ABA BORA ◯ JIMMA ◯ WEST SHEWA ◯ NORTH SHEWA ◯ EAST SHEWA ◯ ARSI ◯ WEST HARARGE ◯ EAST HARARGE ◯ BALE ◯ SOUTH WEST SHEWA ◯ GUJI ◯ ADAMA SPECIAL ◯ WEST ARSI ◯ KELEM WELLEGA ◯ HORO GUDRU WELLEGA ◯ Shinile ◯ Jijiga ◯ Liben ◯ METEKEL ◯ ASOSA ◯ PAWE SPECIAL ◯ GURAGE ◯ HADIYA ◯ KEMBATA TIBARO ◯ SIDAMA ◯ GEDEO ◯ WOLAYITA ◯ SOUTH OMO ◯ SHEKA ◯ KEFA ◯ GAMO GOFA ◯ BENCH MAJI ◯ AMARO SPECIAL ◯ DAWURO ◯ SILTIE ◯ ALABA SPECIAL ◯ HAWASSA CITY ADMINISTRATION ◯ AGNEWAK ◯ MEJENGER ◯ HARARI ◯ AKAKI KALITY ◯ NEFAS SILK-LAFTO ◯ KOLFE KERANIYO 2 ◯ GULELE ◯ LIDETA ◯ KIRKOS-SUB CITY ◯ ARADA ◯ ADDIS KETEMA ◯ YEKA ◯ BOLE ◯ DIRE DAWA filter_list=${level1} ◯ TAHTAY ADIYABO ◯ MEDEBAY ZANA ◯ TSELEMTI ◯ SHIRE ENIDASILASE/TOWN/ ◯ AHIFEROM ◯ ADWA ◯ TAHTAY MAYCHEW ◯ NADER ADET ◯ DEGUA TEMBEN ◯ ABIYI ADI/TOWN/ ◯ ADWA/TOWN/ ◯ AXUM/TOWN/ ◯ SAESI TSADAMBA ◯ KLITE -

World Bank Document
Sample Procurement Plan (Text in italic font is meant for instruction to staff and should be deleted in the final version of the PP) Public Disclosure Authorized (This is only a sample with the minimum content that is required to be included in the PAD. The detailed procurement plan is still mandatory for disclosure on the Bank’s website in accordance with the guidelines. The initial procurement plan will cover the first 18 months of the project and then updated annually or earlier as necessary). I. General 1. Bank’s approval Date of the procurement Plan: Updated Procurement Plan, M 2. Date of General Procurement Notice: Dec 24, 2006 Public Disclosure Authorized 3. Period covered by this procurement plan: The procurement period of project covered from year June 2010 to December 2012 II. Goods and Works and non-consulting services. 1. Prior Review Threshold: Procurement Decisions subject to Prior Review by the Bank as stated in Appendix 1 to the Guidelines for Procurement: [Thresholds for applicable procurement methods (not limited to the list below) will be determined by the Procurement Specialist /Procurement Accredited Staff based on the assessment of the implementing agency’s capacity.] Public Disclosure Authorized Procurement Method Prior Review Comments Threshold US$ 1. ICB and LIB (Goods) Above US$ 500,000 All 2. NCB (Goods) Above US$ 100,000 First contract 3. ICB (Works) Above US$ 15 million All 4. NCB (Works) Above US$ 5 million All 5. (Non-Consultant Services) Below US$ 100,000 First contract [Add other methods if necessary] 2. Prequalification. Bidders for _Not applicable_ shall be prequalified in accordance with the provisions of paragraphs 2.9 and 2.10 of the Public Disclosure Authorized Guidelines. -

In Search of Shelter the Case of Hawassa, Ethiopia
In search of shelter The case of Hawassa, Ethiopia Emma Grant, Gemechu Desta, Yeraswork Admassie, Faraz Hassan, Sophie Stevens and Meheret Ayenew Working Paper Urban Keywords: January 2020 Urbanisation, Informal Settlements, Urban Poverty, Housing About the authors Emma Grant, senior expert, Social Development Direct Gemechu Desta, executive director, Econvalue Consult Yeraswork Admassie, former associate professor of sociology, Addis Ababa University Faraz Hassan, senior urban specialist, Social Development Direct Sophie Stevens, principal consultant, Social Development Direct Meheret Ayenew, senior public policy researcher Acknowledgements With special thanks to Kussia Bekele, senior civil society advisor and research assistant. All photos were taken by members of the Ethiopia research team. The research was funded by the UK Department for International Development’s East Africa Research Fund (EARF) and contributed to the EARF’s research programme: Shaping East African Cities as Systems to Work Better for All. This material has been funded by UK aid from the UK government. However, the views expressed do not necessarily reflect the UK government’s official policies. Produced by IIED’s Human Settlements group The Human Settlements Group works to reduce poverty and improve health and housing conditions in the urban centres of Africa, Asia and Latin America. It seeks to combine this with promoting good governance and more ecologically sustainable patterns of urban development and rural-urban linkages. About Econvalue Consult Econvalue Consult offers advanced policy research expertise on a range of social and economic topics. About Social Development Direct Social Development Direct (SDDirect) provides high-quality, innovative and expert social development assistance and research services. Published by IIED, January 2020 Grant, E, Desta, G, Admassie, Y, Hassan, F, Stevens, S and Ayenew, M (2019) In search of shelter: the case of Hawassa, Ethiopia. -

Study of Seroprevalence and Associated Risk Factors Of
ary Scien in ce r te & e T V e Malicha, et al., J Vet Sci Technol 2017, 8:5 f c h o Journal of Veterinary Science & n n l o o a a DOI: 10.4172/2157-7579.1000471 l l n n o o r r g g u u y y o o J J Technology ISSN: 2157-7579 Research Article Open Access Study of Seroprevalence and Associated Risk Factors of Contagious Bovine Pleuropneumonia in Sidama Zone, Southern Ethiopia Gelgelo Malicha1, Sisay Alemu1*, Fasil Aklilu2 and Ashebr Abraha1 1College of Veterinary Medicine, Haramaya University, P.O. Box 138, Dire Dawa, Ethiopia 2National Animal Health Diagnostic & Investigation Center (NAHDIC), P.O. Box 04, Sebeta, Ethiopia *Corresponding author: Sisay Alemu, Haramaya University College of Veterinary Medicine, P.O. Box 138, Dire Dawa, Ethiopia, Tel: +251-25-5530334; E-mail: [email protected] Rec date: May 31, 2017; Acc date: September 18, 2017; Pub date: September 19, 2017 Copyright: © 2017 Malicha G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Contagious Bovine Pleuropneumonia (CBPP), a disease contributes significantly to cattle morbidity and mortality, remains the most important infectious disease of cattle in Ethiopia. Hence, a cross-sectional study was carried out in nine districts of Sidama Zone, to estimate animal level seroprevalence of CBPP in cattle, and to assess risk factors associated with its occurrence using competitive enzyme linked immunosorbent assay (c-ELISA). -

SITUATION ANALYSIS of CHILDREN and WOMEN: Southern Nations, Nationalities,And People
SITUATION ANALYSIS OF CHILDREN AND WOMEN: Southern Nations, Nationalities,and People SITUATION ANALYSIS OF CHILDREN AND WOMEN: Southern Nations, Nationalities,and People This briefing note covers several issues related to child well-being in Southern Nations, Nationalities and Peoples Region (SNNPR). It builds on existing research and the inputs of UNICEF Ethiopia sections and partners.1 It follows the structure of the Template Outline for Regional Situation Analyses. 1Most of the data included in this briefing note comes from the Ethiopia Demographic and Health Survey (EDHS), Household Consumption and Expenditure Survey (HCE), Education Statistics Annual Abstract (ESAA) and Welfare Monitoring Survey (WMS) so that a valid comparison can be made with the other regions of Ethiopia. SITUATION ANALYSIS OF CHILDREN AND WOMEN: SOUTHERN NATIONS, NATIONALITIES,AND PEOPLE 4 1 THE DEVELOPMENT CONTEXT SNNPR is the third largest region in Ethiopia in terms of population, and is located in the south west of the country. Its estimated population is approximately 20 million people, which makes up 20 per cent of the Ethiopian population.2 The region is one of the most populous parts of Ethiopia, with a density of approximately 151 people per square kilometre. Central SNNPR is particularly highly populated.3 Like elsewhere in the country, the population is young: 14 per cent is under 5 years of age and 47 per cent is between 0 and 17 years of age.4 The total fertility rate (women, aged 15-49) is 4.4, just below the national average of 4.6. The trend analysis -

Explanatory Notes
GEOLOGICAL HAZARDS AND ENGINEERING GEOLOGY MAPS OF DILA NB 37-6 EXPLANATORY NOTES Habtamu Eshetu (Chief Compiler) Vladislav Rapprich (Editor) The Main Project Partners The Czech Development Agency (CzDA) cooperates with the Ministry of Foreign Affairs on the establishment of an institutional framework of Czech development cooperation and actively participates in the creation and financing of development cooperation programs between the Czech Republic and partner countries. www.czda.cz The Geological Survey of Ethiopia (GSE) is accountable to the Ministry of Mines and Energy, collects and assesses geology, geological engineering and hydrogeology data for publication. The project beneficiary. www.gse.gov.et The Czech Geological Survey collects data and information on geology and processes it for political, economical and environmental management. The main contractor. www.geology.cz AQUATEST a.s. is a Czech consulting and engineering company in water management and environmental protection. The main aquatest subcontractor. www.aquatest.cz Copyright © 2014 Czech Geological Survey, Klarov 3, 118 21 Prague 1, Czech Republic First edition AcknowledgmentAcknowledgment Fieldwork and primary compilation of the map and explanatory notes was done by a team from the Geological Survey of Ethiopia (GSE) consisting of staff from the Geo Hazard Investigation Directorate, Groundwater Resources Assessment Directorate and Czech experts from AQUATEST a.s. and the Czech Geological Survey in the framework of the Czech Development Cooperation Program. We would like to thank the SNNPR Regional Water Bureau, the Dila, Sidamo and Sodo- Woleita Zone Administrations, Water, Mines and Energy offices for their hospitality, guidance and relevant data delivery. The team is grateful to the management of the Geological Survey of Ethiopia, particularly to Director General (GSE) Mr. -

World Bank Document
Sample Procurement Plan (Text in italic font is meant for instruction to staff and should be deleted in the final version of the PP) Public Disclosure Authorized (This is only a sample with the minimum content that is required to be included in the PAD. The detailed procurement plan is still mandatory for disclosure on the Bank’s website in accordance with the guidelines. The initial procurement plan will cover the first 18 months of the project and then updated annually or earlier as necessary). I. General 1. Bank’s approval Date of the procurement Plan: Updated Procurement Plan, M 2. Date of General Procurement Notice: Dec 24, 2006 3. Period covered by this procurement plan: The procurement period of project covered from year June 2010 to December 2012 Public Disclosure Authorized II. Goods and Works and non-consulting services. 1. Prior Review Threshold: Procurement Decisions subject to Prior Review by the Bank as stated in Appendix 1 to the Guidelines for Procurement: [Thresholds for applicable procurement methods (not limited to the list below) will be determined by the Procurement Specialist /Procurement Accredited Staff based on the assessment of the implementing agency’s capacity.] Procurement Method Prior Review Threshold Comments US$ 1. ICB and LIB (Goods) Public Disclosure Authorized Above US$ 500,000 All 2. NCB (Goods) Above US$ 100,000 First contract 3. ICB (Works) Above US$ 15 million All 4. NCB (Works) Above US$ 5 million All 5. (Non-Consultant Services) Below US$ 100,000 First contract [Add other methods if necessary] 2. Prequalification. Bidders for _Not applicable_ shall be prequalified in accordance with the provisions of paragraphs 2.9 and 2.10 of the Guidelines. -

Context Analysis: Close-To- Community Providers in Ethiopia
CONTEXT ANALYSIS: CLOSE-TO- COMMUNITY PROVIDERS IN ETHIOPIA ASCHENAKI ZERIHUN, METASEBIA ADMASSU, OLIVIA TULLOCH, MARYSE KOK, DANIEL GEMECHU DATIKO March 2014 Contact Details: Daniel Gemechu Datiko Hidasse Hulentenawi Agelglot Yebego Adragot Mahiber (HHA-YAM), PO Box 303, Hawassa, South Ethiopia Telephone: +251 468209473 Fax: +251 468209473 www.reachoutconsortium.org www.twitter.com/REACHOUT_Tweet Consortium members: James P Grant School of Public Health, BRAC Institute of Global Health, BRAC University (Bangladesh) HHA-YAM (Ethiopia) Eijkman Institute of Molecular Biology (Indonesia) LVCT Health (Kenya) REACH Trust (Malawi) University Eduardo Mondlane (Mozambique) Royal Tropical Institute (KIT, the Netherlands) Liverpool School of Tropical Medicine (LSTM, the UK) Acknowledgements: The investigators would like to acknowledge the following organizations and individuals for their unlimited support and help for the realization of this study. We would like to thank the European Union for its financing and support. This document reflects only the authors’ views, and the European Union is not liable for any use that may be made of the information contained herein. We would like to acknowledge the effort of Maryse Kok, researcher from the Royal Tropical Institute (KIT) in the Netherlands and Dr. Olivia Tulloch, Research Manager for REACHOUT from the Liverpool School of Tropical Medicine for their unlimited support, guidance, comments and critiques throughout the whole process of this study. Our gratitude goes to Mr. Alemu Tamiso, lecturer at Arbaminch University, and Mr. Mehretu Belayneh, lecturer at Hawassa University, for their commitment and support during qualitative data collection, transcription and translation. We are grateful to the Health Bureau of South Region, Sidama Zone Health Department, Save the Children and the Integrated Family Health Program regional offices for providing the necessary information and data for the desk study. -

Ethiopia: SNNP Region Administrative Map (As of 15 Aug 2017)
Ethiopia: SNNP region administrative map (as of 15 Aug 2017) ! ! ! ! ! ! ! ! ! Suten ! ! ! ! ! ! Inge Sodo ! ! !Bui ! ! WelikiteKebena Abeshege ! Kokir Gedbano ! ! Kela ! ! Muhur Na Ak!lil ! Gubire ! ! ! Cheha Agena ! Imdibir! ! Ezha Me!skan ! ! Inseno ! Gonichire ! ! ! Kibet Qewaqoto! Koshe ! ! ! ! ! ! ! Enemorina Eaner Alicho Woriro ! Gumer Mareko ! Selti ! ! Areket Alkeso town ! ! ! ! ! ! Geta Kose Tora ! Fofa ! Werabe ! ! ! Dinkela ! ! Sayilem! ! ! ! ! Yadota Geja Endiguagn Yem SP Woreda ! Dalocha ! Misrak Azenet Berbere ! ! ! ! Misha !LERA Dalocha Masha ! Wilb!areg Gibe ! ! Mierab Azenet Berbere ! ! Lanfero ! Homec!ho ! ! Fonqo town ! Mito ! GAMBELA Gesha (Deka) Kondo GECHA TOWN ! Analemmo ! ! !Deka ! Doesha !Belesa town ! Alem Gebeya Anderacha Getawa Gembora ! ! Limu ! ! Bonosha Sankura ! ! ! Lisana town Jajira Shashogo Gimbichu! ! Hufa ! ! ! Diri Soro ! Gojeb Bita (Big) Gimbo Doya Gena Jacho A!nigach!a ! Alaba SP Woreda ! ! ! Daniboya Wishiwishi Dune Kulito ! Kaka Idget ! Bita Genet ! OROMIA Kelata Mudula Hobichaka ! ! Bonga ! ! ! ! ! Yeki ! Menjiwo ! Chena Tembaro Ke!diada Gambela TEPI TOWN Hadero !TubitoKacha Bira ! ! ! !Adilo Chda Idge T!unito ! Legend WACHA ! ! Terche Misrak Badawacho ! Gena Bosa Chiri BOMIBE 01 ! ! ! ! !Karewo ! Mierab Badawacho ! Ameya P ! Tocha Tocha Edget Boloso Bombe Sheka Tulo ! Regional capital ! Waka ! Semen Bench Alem Gena ! ! ! ! Mehal Sheko Mareka Boloso SoreDamot Pulasa Hawassa Zuria PWondo-Ge! net Gesa ! ! Shanito Hawasa Town ! ! ! ! Shama Chuko Shay Bench ! Bitena Town Mizan Aman ! ! Tula ! Damot -
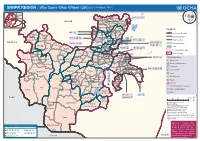
SNNPR REGION : Who Does What Where (3W) (As of 01 March 2012)
(as of 01 March 2012) SNNPR REGION : Who Does What Where (3W) Tigray Afar Amhara Sodo! ECS: a cç Benshangul Gumuz KebenaKokir Gedbano Dire Dawa Abeshege Addis Ababa Hareri Gambela Oromia Oromia Muhur Na Aklil Somali Cheha SNNPR Gurage Ezha Meskan Alicho Woriro Enemorina Eaner Gumer Selti Mareko Yem Geta Selti Legend Sayilem ! IRC: ç Endiguagn Dalocha Yem SP Wor!eda International Boundary Masha Gibe Misha Wilbareg Lanfero Regional Boundary Sheka Gesha (Deka) ECS: ah ç HadiyaAnalemmo ! Anderacha Getawa Gembora ! Sankura LVIA: a 4 l Zonal Boundary ! Limu Gambella Shash! ogo LVIA: a 4 l Plan Int.: : h Soro Woreda Boundary Gimbo Anigacha Alaba SP Woreda SC UK: h Bita (Big) ! Lake Dune Daniboy! a Alaba KT Plan Int.: h IMC: î h ç Yeki Chena Menjiwo Tembaro Keffa Kacha BiraKediada Gambela No Intervention/No Data Gena Bosa Misrak Badawacho Tocha Boloso Bom! be Other Region Sheka Tulo Wondo-Genet Semen Bench Boloso sore Awassa Zuria Mareka A! wasa Town Dawro Damot Gale Plan Int.: d Clusters/Sectors Ela (Konta) SP Woreda Kindo Koysha Diguna Fango ! Malga Gurafereda Debub BenchShay Bench Cheta Boricha Agriculture Decha Esira Damot Sore a Konta Loma Bosa Sodo ZuriaDamot Weydie Shebe DinoGorche Wolayita Dale : Disaster Management Menit Goldiye Kindo Dida Ofa Humbo Wonosho Arbe Gonna d Education ! Loka-Abaya ! Bursa Sidama ACF: aîlf Chuko 4 Environment Melekoza Kucha Boreda Bensa Menit Shasha ! Hulla Denibu Gofa Dara Bona Zu! ria ç Chire î Food Dila Zuria Bero BasketoGeze Gofa h Aroresa l Food Security Zala Mirab Abaya Wenago Basketo SP Woreda DaramaloDita