Antioxidant Biflavonoids from Garcinia Madruno
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Postharvest Quality of Achachairú (Garcinia Gardneriana) Stored at Ambient Temperature1,2 Marlyn Cotty-Más3, Rosa N
Postharvest quality of achachairú (Garcinia gardneriana) stored at ambient temperature1,2 Marlyn Cotty-Más3, Rosa N. Chávez-Jáuregui4 and Linda Wessel-Beaver5 J. Agric. Univ. P.R. 103(2):155-172 (2019) ABSTRACT Achachairú is a tropical fruit that is being evaluated for its potential as a new fruit crop for Puerto Rico. More information is needed concerning postharvest aspects of this fruit. In this paper we describe the physical and chemical characteristics of achachairú during storage at ambient temperature. During each of three harvest years, 75 to 100 fruits were harvested, washed and dried, then divided into five groups. Each group was placed in an open cardboard box and randomly assigned to one of five storage treatments (0, 5, 10, 15, and 20 days at 25 ± 2 °C, 75 ± 5% relative humidity). Physical and chemical properties, including sugar content, were determined for each storage period. Sensory panels evaluated fruits after 0 and 15 days of storage. Over the 20-day storage period fruit weight, size (length and diameter), firmness and pulp weight decreased by 1.5% to 2.5% per day. The rating of external fruit appearance (evaluated on a hedonic scale) deteriorated in a curvilinear fashion over time, initially changing little, and then showing increased deterioration (dark spots and wrinkling) starting at 15 days post storage. Total soluble solids (°Brix) (TSS) increased over time by 0.8 to 1.6% per day, while total titratable acid (TTA) decreased 1.4 to 3.0% per day, resulting in an increase of the sugar-acid ratio (TSS/TTA) of 2.1 to 12.4% per day over 20 days of storage. -

Chec List What Survived from the PLANAFLORO Project
Check List 10(1): 33–45, 2014 © 2014 Check List and Authors Chec List ISSN 1809-127X (available at www.checklist.org.br) Journal of species lists and distribution What survived from the PLANAFLORO Project: PECIES S Angiosperms of Rondônia State, Brazil OF 1* 2 ISTS L Samuel1 UniCarleialversity of Konstanz, and Narcísio Department C.of Biology, Bigio M842, PLZ 78457, Konstanz, Germany. [email protected] 2 Universidade Federal de Rondônia, Campus José Ribeiro Filho, BR 364, Km 9.5, CEP 76801-059. Porto Velho, RO, Brasil. * Corresponding author. E-mail: Abstract: The Rondônia Natural Resources Management Project (PLANAFLORO) was a strategic program developed in partnership between the Brazilian Government and The World Bank in 1992, with the purpose of stimulating the sustainable development and protection of the Amazon in the state of Rondônia. More than a decade after the PLANAFORO program concluded, the aim of the present work is to recover and share the information from the long-abandoned plant collections made during the project’s ecological-economic zoning phase. Most of the material analyzed was sterile, but the fertile voucher specimens recovered are listed here. The material examined represents 378 species in 234 genera and 76 families of angiosperms. Some 8 genera, 68 species, 3 subspecies and 1 variety are new records for Rondônia State. It is our intention that this information will stimulate future studies and contribute to a better understanding and more effective conservation of the plant diversity in the southwestern Amazon of Brazil. Introduction The PLANAFLORO Project funded botanical expeditions In early 1990, Brazilian Amazon was facing remarkably in different areas of the state to inventory arboreal plants high rates of forest conversion (Laurance et al. -

Systematics and Floral Evolution in the Plant Genus Garcinia (Clusiaceae) Patrick Wayne Sweeney University of Missouri-St
University of Missouri, St. Louis IRL @ UMSL Dissertations UMSL Graduate Works 7-30-2008 Systematics and Floral Evolution in the Plant Genus Garcinia (Clusiaceae) Patrick Wayne Sweeney University of Missouri-St. Louis Follow this and additional works at: https://irl.umsl.edu/dissertation Part of the Biology Commons Recommended Citation Sweeney, Patrick Wayne, "Systematics and Floral Evolution in the Plant Genus Garcinia (Clusiaceae)" (2008). Dissertations. 539. https://irl.umsl.edu/dissertation/539 This Dissertation is brought to you for free and open access by the UMSL Graduate Works at IRL @ UMSL. It has been accepted for inclusion in Dissertations by an authorized administrator of IRL @ UMSL. For more information, please contact [email protected]. SYSTEMATICS AND FLORAL EVOLUTION IN THE PLANT GENUS GARCINIA (CLUSIACEAE) by PATRICK WAYNE SWEENEY M.S. Botany, University of Georgia, 1999 B.S. Biology, Georgia Southern University, 1994 A DISSERTATION Submitted to the Graduate School of the UNIVERSITY OF MISSOURI- ST. LOUIS In partial Fulfillment of the Requirements for the Degree DOCTOR OF PHILOSOPHY in BIOLOGY with an emphasis in Plant Systematics November, 2007 Advisory Committee Elizabeth A. Kellogg, Ph.D. Peter F. Stevens, Ph.D. P. Mick Richardson, Ph.D. Barbara A. Schaal, Ph.D. © Copyright 2007 by Patrick Wayne Sweeney All Rights Reserved Sweeney, Patrick, 2007, UMSL, p. 2 Dissertation Abstract The pantropical genus Garcinia (Clusiaceae), a group comprised of more than 250 species of dioecious trees and shrubs, is a common component of lowland tropical forests and is best known by the highly prized fruit of mangosteen (G. mangostana L.). The genus exhibits as extreme a diversity of floral form as is found anywhere in angiosperms and there are many unresolved taxonomic issues surrounding the genus. -

Validação Do Nome Garcinia Leptophylla (Clusiaceae)
Bol. Mus. Para. Emílio Goeldi. Cienc. Nat., Belém, v. 13, n. 1, p. 19-23, jan.-abr. 2018 Validation of the name Garcinia leptophylla (Clusiaceae) Validação do nome Garcinia leptophylla (Clusiaceae) Volker BittrichI, Lucas C. MarinhoII ICampinas, São Paulo, Brasil IIUniversidade Estadual de Feira de Santana. Feira de Santana, Bahia, Brasil Abstract: During the continuous production of the “Catálogo de plantas e fungos do Brasil” (2010), some new combinations under Garcinia were proposed; however, they are invalid, since the respective basionyms were not indicated. Here, we propose the validation of the name Garcinia leptophylla Bittrich, a new name proposed for Rheedia longifolia Planch. & Triana. A lectotype for the name is also designated. Keywords: Brazilian flora. Amazon forest. Nomenclature. Rheedia longifolia. Taxonomy. Resumo: Durante a produção do “Catálogo de plantas e fungos do Brasil” (2010), algumas novas combinações foram propostas em Garcinia, entretanto, elas são consideradas inválidas, uma vez que seus respectivos basiônimos não foram indicados. Neste artigo, propomos a validação do nome Garcinia leptophylla Bittrich, um novo nome apresentado para Rheedia longifolia Planch. & Triana, bem como designamos um lectótipo. Palavras-chave: Flora brasileira. Floresta amazônica. Nomenclatura. Rheedia longifolia. Taxonomia. BITTRICH, V. & L. C. MARINHO, 2018. Validation of the name Garcinia leptophylla (Clusiaceae). Boletim do Museu Paraense Emílio Goeldi. Ciências Naturais 13(1): 19-23. Autor para correspondência: Lucas C. Marinho. Universidade Estadual de Feira de Santana. Programa de Pós-Graduação em Botânica. Avenida Transnordestina – Novo Horizonte. Feira de Santana, BA, Brasil. CEP 44036-900 ([email protected]). Recebido em 03/11/2017 Aprovado em 07/11/2017 Responsabilidade editorial: Fernando da Silva Carvalho Filho 19 Validation of the name Garcinia leptophylla (Clusiaceae) INTRODUÇÃO were proposed [i.e. -

Cyrtophora Citricola (Arachnida: Araneae: Araneidae)1 G
EENY-535 A Colonial Tentweb Orbweaver scientific name: Cyrtophora citricola (Arachnida: Araneae: Araneidae)1 G. B. Edwards2 Introduction Distribution Few species of spiders can be considered truly social, but Cyrtophora citricola is widespread in subtropical and more species, particularly web-building spiders, live in tropical areas of Asia, Africa, Australia, and in the warm close proximity to one another, potentially gaining benefits coastal Mediterranean areas of Europe (Blanke 1972, by this association. Among these benefits are sharing of Leborgne et al. 1998). It was found in Colombia in 1996 frame threads (Kullman 1959), improved defense against (Levi 1997, Pulido 2002), the Dominican Republic in 1999 predators and parasites (Cangialosi 1990), improved prey (Alayón 2001), Florida in 2000, and Cuba in 2003 (Alayón capture efficiency (Rypstra 1979, Uetz 1989), and greater 2003). Survey work was performed August 2000, April egg production (Smith 1983). 2001, and July 2002 to document the spread of the species in Florida. The survey was focused on canal bridges because Of the three main types of aggregative behaviors exhibited Cyrtophora citricola has a tendency to make its webs on by spiders, the one with the least social interaction involves the guardrails of canal bridges (Figure 6). The survey work individuals making and maintaining their own webs within in 2000 established a preliminary periphery of infestation a colonial matrix of interconnected webs (Buskirk 1975). in a narrow band from west of Homestead to northeast of One such species, which has become highly successful Homestead. through a lifestyle of colonial aggregation, is the orbweaver Cyrtophora citricola Forskål. This species is known as a To date, the known distribution of Cyrtophora citricola in tentweb spider in Africa (Dippenaar-Schoeman and Jocqué Florida is a parallelogram-shaped area from east of the 1997). -

Tropical Agricultural Research and Higher Education Center (CATIE)
Policies, programmes and activities related to biodiversity for food and agriculture Reports from international instruments and organizations 1. Contact information Name and position of respondent Leida Mercado, Director of Research and Development Division at CATIE Name of organization Tropical Agricultural Research and Higher Education Center (CATIE) E-mail of organization [email protected], [email protected] Geographical coverage of your organization Latin America Caribbean 2. Components of biodiversity for food and agriculture covered by your organization Note: For a complete definition refer to Annex 1 of: http://www.fao.org/nr/cgrfa/biodiversity/guidelines/en/ Sectoral genetic resources for food and agriculture Animal genetic resources Aquatic genetic resources Forest genetic resources Plant genetic resources Associated biodiversity of relevance to food and agriculture Micro-organisms (including bacteria, viruses, protists and fungi) Invertebrates (including insects, spiders, worms) Vertebrates (including amphibians, reptiles and non-domesticated birds and mammals) Wild and cultivated terrestrial and aquatic plants other than crop wild relatives Page 1 of 10 Please provide details on the components of biodiversity for food and agriculture involved (species, breeds, varieties): Germoplasm collection includes vegetables seed in cold storage and orthodox seeds like coffee cacao, peach palm and others plants in the field: Allium cepa (1), Amaranthus caudatus (10), Amaranthus cruentus (6), Amaranthus hybridus (16), Amaranthus hypochondriacus (2), Amaranthus sp. (232), Chenopodium berlandieri (2), Chenopodium quinoa (4), Coriandrum sp. (1), Vernonia galamensi (1), Benincasa hispida (1), Benincasa cerífera (1), Cionosicyos sp. (4), Citrullus lanatus (6), Citrullus sp. (3), Cucumis anguria (2), Cucumis melo (13), Cucumis metuliferus (1), Cucumis sativus (8), Cucumis sp. -

Phenology of Tree Species of the Osa Peninsula and Golfo Dulce Region, Costa Rica 547-555 © Biologiezentrum Linz/Austria; Download Unter
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Stapfia Jahr/Year: 2008 Band/Volume: 0088 Autor(en)/Author(s): Lobo Jorge A., Aguilar Reinaldo, Chacon Eduardo, Fuchs Eric Artikel/Article: Phenology of tree species of the Osa Peninsula and Golfo Dulce region, Costa Rica 547-555 © Biologiezentrum Linz/Austria; download unter www.biologiezentrum.at Phenology of tree species of the Osa Peninsula and Golfo Dulce region, Costa Rica Fenologìa de especies de árboles de la Península de Osa y la región de Golfo Dulce, Costa Rica J orge L OBO,Reinaldo A GUILAR,Eduardo C HACÓN &Eric F UCHS Abstract: Data on leafing, flowering and fruiting phenology are presented for 74 tree species from the Osa Peninsula and Golfo Dulce, SE Costa Rica. Data was gathered from direct observations of phenological events from 1989 to 2007 from marked and unmarked trees in different sites in the Osa Peninsula. Flowering and fruiting peaks were observed during the dry season (Decem- ber to March), with a second fruiting peak observed in the middle of the rainy season. We observed a large diversity in pheno- logical patterns, but similar numbers of species flowered and produced fruit in the dry and rainy season. A reduction in the num- ber of species in reproduction occurs in the months with the highest precipitation (August to October). Comparison of Osa phe- nological data with the phenology of wet and dry forests from Costa Rica and Panamá showed some similarities in the timing of phenological events. However, Osa species display a shift in phenological events with an earlier onset of flower and fruit produc- tion in comparison with other sites. -
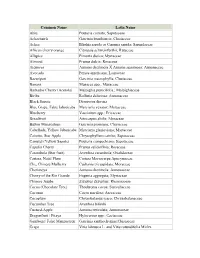
Common Name Latin Name Abiu
Common Name Latin Name Abiu Pouteria caimito; Sapotaceae Achachairú Garcinia brasiliensis; Clusiaceae Ackee Blighia sapida or Cupania sapida; Sapindaceae African cherry orange Citropsis schweinfurthii; Rutaceae Allspice Pimenta dioica; Myrtaceae Almond Prunus dulcis; Rosaceae Atemoya Annona cherimola X Annona squamosa; Annonaceae Avocado Persea americana; Lauraceae Bacuripari Garcinia macrophylla; Clusiaceae Banana Musacea spp.; Musaceae Barbados Cherry (Acerola) Malpighia punicifolia.; Malpighiaceae Biriba Rollinia deliciosa; Annonaceae Black Sapote Diospyros digyna Blue Grape, False Jaboticaba Myrciaria vexator; Myrtaceae Blueberry Vaccinium spp.; Ericaceae Breadfruit Artocarpus altilis; Moraceae Button Mangosteen Garcinia prainiana; Clusiaceae Cabelluda, Yellow Jaboticaba Myrciaria glazioviana; Myrtaceae Caimito, Star Apple Chrysophyllum cainito; Sapotaceae Canistel (Yellow Sapote) Pouteria campechiana; Sapotaceae Capulin Cherry Prunus saliciofloia; Rosaceae Carambola (Star fruit) Averrhoa carambola; Oxalidaceae Carissa, Natal Plum Carissa Macrocarpa;Apocynaceae Che, Chinese Mulberry Cudrania tricuspidata; Moraceae Cherimoya Annona cherimola; Annonaceae Cherry of the Rio Grande Eugenia aggregata; Myrtaceae Chinese Jujube Ziziphus Zizyphus; Rhamnaceae Cocoa (Chocolate Tree) Theobroma cocoa; Sterculiaceae Coconut Cocos nucifera; Arecaceae Cocoplum Chrysobalanus icaco; Chrysobalanaceae Cucumber Tree Averrhoa bilimbi Custard-Apple Annona reticulata; Annonaceae Dragonfruit / Pitaya Hylocereus spp.; Cactaceae Gamboge/ False Mangosteen Garcinia -

Antioxidant and Antimicrobial Activity Assessment of Methanol Extract of Garcinia Cowa Leaves
Antioxidant and Anti-microbial Activity Assessment of Methanol Extract of Garcinia cowa Leaves A Dissertation submitted to the Department of Pharmacy, East West University, in partial fulfillment of the requirements for the degree of Bachelor of Pharmacy. Submitted By: Farhena Afrose Tanha ID: 2013-1-70-038 Department of Pharmacy East West University Declaration I, Farhena Afrose Tanha hereby declare that this dissertation, entitled 'Antioxidant and Antimicrobial Assesment of Methanol Extract of Garcinia cowa Leaves ' submitted to the Department of Pharmacy, East West University, in the partial fulfillment of the requirement for the degree of Bachelor of Pharmacy (Honors) is a genuine & authentic research work carried out by me. The contents of this dissertation, in full or in parts, have not been submitted to any other Institute or University for the award of any Degree or Diploma or Fellowship. -------------------------------------------- Farhena Afrose Tanha ID: 2013-1-70-038 Department of Pharmacy East West University Aftabnagar, Dhaka 2 CERTIFICATION BY THE SUPERVISOR This is to certify that the dissertation, entitled 'Antioxidant and Antimicrobial Investigations of Methanol Extract of Garcinia cowa leaves' is a research work carried out by Farhena Afrose (ID: 2013-1-70-038) in 2017, under the supervision and guidance of me, in partial fulfillment of the requirement for the degree of Bachelor of Pharmacy. The thesis has not formed the basis for the award of any other degree/diploma/fellowship or other similar title to any candidate of any university. --------------------------------------- Nazia Hoque Assistant Professor Department of Pharmacy, East West University, Dhaka 3 ENDORSEMENT BY THE CHAIRPERSON This is to certify that the dissertation, entitled is a research work carried out 'Antioxidant and Antimicrobial Investigations Of Methanol Extract Of Garcinia cowa stem' by Farhena Afrose Tanha (ID: 2013-1-70-038), under the supervision and guidance of Ms. -

Systematics and Floral Evolution in the Plant Genus Garcinia (Clusiaceae)
University of Missouri, St. Louis IRL @ UMSL Dissertations UMSL Graduate Works 7-30-2008 Systematics and Floral Evolution in the Plant Genus Garcinia (Clusiaceae) Patrick Wayne Sweeney University of Missouri-St. Louis Follow this and additional works at: https://irl.umsl.edu/dissertation Part of the Biology Commons Recommended Citation Sweeney, Patrick Wayne, "Systematics and Floral Evolution in the Plant Genus Garcinia (Clusiaceae)" (2008). Dissertations. 539. https://irl.umsl.edu/dissertation/539 This Dissertation is brought to you for free and open access by the UMSL Graduate Works at IRL @ UMSL. It has been accepted for inclusion in Dissertations by an authorized administrator of IRL @ UMSL. For more information, please contact [email protected]. SYSTEMATICS AND FLORAL EVOLUTION IN THE PLANT GENUS GARCINIA (CLUSIACEAE) by PATRICK WAYNE SWEENEY M.S. Botany, University of Georgia, 1999 B.S. Biology, Georgia Southern University, 1994 A DISSERTATION Submitted to the Graduate School of the UNIVERSITY OF MISSOURI- ST. LOUIS In partial Fulfillment of the Requirements for the Degree DOCTOR OF PHILOSOPHY in BIOLOGY with an emphasis in Plant Systematics November, 2007 Advisory Committee Elizabeth A. Kellogg, Ph.D. Peter F. Stevens, Ph.D. P. Mick Richardson, Ph.D. Barbara A. Schaal, Ph.D. © Copyright 2007 by Patrick Wayne Sweeney All Rights Reserved Sweeney, Patrick, 2007, UMSL, p. 2 Dissertation Abstract The pantropical genus Garcinia (Clusiaceae), a group comprised of more than 250 species of dioecious trees and shrubs, is a common component of lowland tropical forests and is best known by the highly prized fruit of mangosteen (G. mangostana L.). The genus exhibits as extreme a diversity of floral form as is found anywhere in angiosperms and there are many unresolved taxonomic issues surrounding the genus. -

Latin America and the Caribbean Regional Synthesis for the State of the World’S Biodiversity for Food and Agriculture
REGIONAL SYNTHESIS REPORTS LATIN AMERICA AND THE CARIBBEAN REGIONAL SYNTHESIS FOR THE STATE OF THE WORLD’S BIODIVERSITY FOR FOOD AND AGRICULTURE LATIN AMERICA AND THE CARIBBEAN REGIONAL SYNTHESIS FOR THE STATE OF THE WORLD’S BIODIVERSITY FOR FOOD AND AGRICULTURE FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS ROME, 2019 Required citation: FAO. 2019. Latin America and the Caribbean Regional Synthesis for The State of the World’s Biodiversity for Food and Agriculture. Rome. https://doi.org/10.4060/ca7125en. The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by FAO in preference to others of a similar nature that are not mentioned. The views expressed in this information product are those of the author(s) and do not necessarily reflect the views or policies of FAO. ISBN 978-92-5-131991-8 © FAO, 2019 Some rights reserved. This work is made available under the Creative Commons Attribution-NonCommercial- ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo/ legalcode/legalcode). Under the terms of this licence, this work may be copied, redistributed and adapted for non-commercial purposes, provided that the work is appropriately cited. -

Structural Diversity of Secondary Metabolites in Garcinia Species
JNTBGRI Diversity of Garcinia species in the Western Ghats: Phytochemical Perspective Chapter 2 Structural diversity of secondary metabolites in Garcinia species A. P. Anu Aravind, Lekshmi N. Menon and K. B. Rameshkumar* Phytochemistry and Phytopharmacology Division Jawaharlal Nehru Tropical Botanic Garden and Research Institute Palode, Thiruvananthapuram-695562, Kerala, India * Corresponding author Abstract Plants of the genus Garcinia produce structurally diverse secondary metabolites such as biflavonoids, xanthones, benzophenones, flavonoids, biphenyls, acyl phloroglucinols, depsidones and terpenoids. The rich diversity in chemical structures made the genus Garcinia attractive for the phytochemists. In addition, several industrial sectors such as cosmetic, food, pharmaceutics, neutraceutics and paints are centered around the genus. The genus is represented by more than 250 species, among which nearly 120 species were subjected to phytochemical investigation. A review of the structural diversity of secondary metabolites of Garcinia species revealed that xanthones are the important class of secondary metabolites, distributed in 74 Garcinia species, followed by benzophenones in 50 species and biflavonoids in 45 species. Biphenyls, acyl phloroglucinols, depsidones and flavonoids are some other interesting group of phenolic compounds in Garcinia species. The present chapter enlists the major phenolic compounds reported from Garcinia species. Keywords: Garcinia, Secondary metabolites, Xanthones, Biflavonoids, Benzophenones Introduction Plants