Revised Guides for Organ Sampling in Rats and Mice
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Human Anatomy (Biology 2) Lecture Notes Updated July 2017 Instructor
Human Anatomy (Biology 2) Lecture Notes Updated July 2017 Instructor: Rebecca Bailey 1 Chapter 1 The Human Body: An Orientation • Terms - Anatomy: the study of body structure and relationships among structures - Physiology: the study of body function • Levels of Organization - Chemical level 1. atoms and molecules - Cells 1. the basic unit of all living things - Tissues 1. cells join together to perform a particular function - Organs 1. tissues join together to perform a particular function - Organ system 1. organs join together to perform a particular function - Organismal 1. the whole body • Organ Systems • Anatomical Position • Regional Names - Axial region 1. head 2. neck 3. trunk a. thorax b. abdomen c. pelvis d. perineum - Appendicular region 1. limbs • Directional Terms - Superior (above) vs. Inferior (below) - Anterior (toward the front) vs. Posterior (toward the back)(Dorsal vs. Ventral) - Medial (toward the midline) vs. Lateral (away from the midline) - Intermediate (between a more medial and a more lateral structure) - Proximal (closer to the point of origin) vs. Distal (farther from the point of origin) - Superficial (toward the surface) vs. Deep (away from the surface) • Planes and Sections divide the body or organ - Frontal or coronal 1. divides into anterior/posterior 2 - Sagittal 1. divides into right and left halves 2. includes midsagittal and parasagittal - Transverse or cross-sectional 1. divides into superior/inferior • Body Cavities - Dorsal 1. cranial cavity 2. vertebral cavity - Ventral 1. lined with serous membrane 2. viscera (organs) covered by serous membrane 3. thoracic cavity a. two pleural cavities contain the lungs b. pericardial cavity contains heart c. the cavities are defined by serous membrane d. -

The Costs and Benefits of Moving to the ICD-10 Code Sets
CHILDREN AND ADOLESCENTS This PDF document was made available from www.rand.org as a public CIVIL JUSTICE service of the RAND Corporation. EDUCATION ENERGY AND ENVIRONMENT Jump down to document HEALTH AND HEALTH CARE 6 INTERNATIONAL AFFAIRS POPULATION AND AGING The RAND Corporation is a nonprofit research PUBLIC SAFETY SCIENCE AND TECHNOLOGY organization providing objective analysis and effective SUBSTANCE ABUSE solutions that address the challenges facing the public TERRORISM AND HOMELAND SECURITY and private sectors around the world. TRANSPORTATION AND INFRASTRUCTURE U.S. NATIONAL SECURITY Support RAND Purchase this document Browse Books & Publications Make a charitable contribution For More Information Visit RAND at www.rand.org Explore RAND Science and Technology View document details Limited Electronic Distribution Rights This document and trademark(s) contained herein are protected by law as indicated in a notice appearing later in this work. This electronic representation of RAND intellectual property is provided for non-commercial use only. Permission is required from RAND to reproduce, or reuse in another form, any of our research documents for commercial use. This product is part of the RAND Corporation technical report series. Reports may include research findings on a specific topic that is limited in scope; present discus- sions of the methodology employed in research; provide literature reviews, survey instruments, modeling exercises, guidelines for practitioners and research profes- sionals, and supporting documentation; -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Methods and Apparatus for Sampling and Analyzing Body Fluid
Europäisches Patentamt *EP001579814A2* (19) European Patent Office Office européen des brevets (11) EP 1 579 814 A2 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.7: A61B 17/32 28.09.2005 Bulletin 2005/39 (21) Application number: 05005734.8 (22) Date of filing: 16.05.1997 (84) Designated Contracting States: (72) Inventors: AT BE CH DE DK ES FI FR GB GR IE IT LI LU MC • Douglas, Joel S. NL PT SE Los Altos Hills, CA 94022 (US) • Roe, Jeffrey N. (30) Priority: 17.05.1996 US 17133 P San Ramon, CA 94583 (US) 14.06.1996 US 19918 P • Radwanski, Ryszard 01.08.1996 US 23658 P Morgan Hill, CA 95037 (US) 03.09.1996 US 25340 P • Duchon, Brent G. 16.09.1996 US 714548 Garden Grove, CA 92845 (US) 17.09.1996 US 710456 08.10.1996 US 727074 (74) Representative: Vossius & Partner Siebertstrasse 4 (62) Document number(s) of the earlier application(s) in 81675 München (DE) accordance with Art. 76 EPC: 97929682.9 / 0 955 909 Remarks: This application was filed on 16 - 03 - 2005 as a (71) Applicant: Roche Diagnostics Operations, Inc. divisional application to the application mentioned Indianapolis, Indiana 46250 (US) under INID code 62. (54) Methods and apparatus for sampling and analyzing body fluid (57) A sampling device (10) for sampling body fluid includes a lancet (12) for making an incision, a capillary tube (18) for drawing up body fluid from the incision, and a test strip (30) affixed to an upper end of the capillary tube (18) for receiving the fluid. -

Anatomy of the Pig
Hands on Workshop: Lecture for Animal Workshop Anatomy of the pig Jong Man Kim1, Hae Won Lee2 Sungkyunkwan University1, Seoul National University2, Korea Introduction The digestive system of swine has anatomic differences from humans. However, the physiology of digestion remains similar to humans. In spite of the anatomic differences, the pig has been used extensively as a gastro- intestinal model. Most of the classical models involving the digestive system have been related to nutritional stud- ies to study digestion of the pig and for studying human digestive phenomenon. More recently endoscopic and laparoscopic surgical models have been developed and used extensively in the swine. The size and function of structures such as the biliary system and pancreatic duct make them amenable for studying human sized equip- ment and biomaterial implants. Surgical modifications have made the intestinal tract amenable to the study of surgical and chronic fistulation procedures. Laparoscopic surgery has replaced many open operations. The procedures described are commonly performed laparoscopically by many general surgeons but require practice. The porcine model is ideal to train surgeons in laparoscopic procedures since porcine anatomy is generally similar to humans with some minor differences. Liver 1. Morphological feature In the human, the liver morphologically consists of 4 lobes; the left, right, quadrate, and caudate lobes al- though the functional anatomy is more important than the morphological one, which is rarely used in clinical field. Unlikely to the human, the porcine liver consists of 5 lobes; the left lateral and medial, right lateral and medial, and caudate lobes. In the ventral view, 4 lobes are seen; the left lateral, left medial, right medial, and right lateral lobes in sequence from left to right. -

Fluid Bodies: an Overview Evanjali Pradhan * *Department of Microbiology, Utkal University, India
OPEN ACCESS Freely available online e a Journal of ISSN: 2168-9873 Applied Mechanical Engineering Editorial Fluid Bodies: An Overview Evanjali Pradhan * *Department of Microbiology, Utkal University, India EDITORIAL Arterial blood sampling, such as radial artery puncture Body fluids, also known as bodily fluids or biofluids, are the liquids Osmosis is a mechanism in which water travels from one that make up the human body. Total body water makes up about compartment of the body to another via semi-permeable cell 60% (60–67%) of the total body weight in lean, stable adult men; it membranes. Osmosis is the diffusion of water over a semi- is slightly lower in women. The amount of body fat is inversely permeable membrane from regions of higher concentration to proportional to the same percentage of fluid compared to body regions of lower concentration along an osmotic gradient. As a weight. For example, a lean 70 kg (160 pound) man has around 42 result, depending on the relative amounts of water and solutes (42-47) litres of water. present in cells and tissues, water can flow into and out of them. Health To ensure normal operation, a proper balance of solutes within and outside of cells must be maintained. Water makes up about The word "body fluid" is most widely used in medical and health 75 percent of the body mass in children, 50–60 percent in adult contexts. Body fluids are known as inherently unclean in current men and women, and as little as 45 percent in the elderly. Since medical, public health, and personal hygiene practises. -

Labinvest201230.Pdf
ANNUAL MEETING ABSTRACTS 81A found between the number of positive biopsies and mortality. Only 6 of 42 diffuse 329 Pathological Features of Adventitial infl ammatory Reaction in C4d+ cases evolved to show DSA and dysfunction. Thirty six of 42 diffuse C4d+ cases Acute Aortic Dissections showed no dysfunction, despite the presence of DSA in 6 of them. Thus interpreted LF Xu, C Miller, AP Burke. University of Maryland Medical Center, Baltimore, MD. as accommodation. Background: Histological reaction in the adventitia to aortic dissections has not been Conclusions: Serial staining of biopsies shows that: 1. Concomitant C4d and C3d well studied. We present histological fi ndings in a series of acute aortic dissections with positivity correlates highly with allograft dysfunction and DSA; 2. Diffuse C4d capillary emphasis regarding dating and infl ammatory reaction. staining alone should not be equated with AMR; 3. Most C4d+ episodes are single Design: We prospectively studied 43 surgically excised acute ascending aortic occurrences and asymptomatic; 4. The presence of C4d staining and DSA without dissections. We evaluated the histological reaction in the media and adventitia adjacent allograft dysfunction may indicate accommodation; 5. Only a small fraction of patients to the acute dissection plane in 4 or more sections of aorta oriented perpendicularly. with C4d staining alone may develop AMR on follow-up. Infl ammation (both degree and type) and stromal reaction were semiquantitated and correlated with duration of symptoms prior to surgical repair. 327 Carbonic Anhydrase IX – Hypoxia Marker in the Aortic Wall Results: Of the 43 cases, there were 31 men (ages 53 ± 14 years) and 13 women (ages Y Sheykin, S Rosen. -

Brain Anatomy
BRAIN ANATOMY Adapted from Human Anatomy & Physiology by Marieb and Hoehn (9th ed.) The anatomy of the brain is often discussed in terms of either the embryonic scheme or the medical scheme. The embryonic scheme focuses on developmental pathways and names regions based on embryonic origins. The medical scheme focuses on the layout of the adult brain and names regions based on location and functionality. For this laboratory, we will consider the brain in terms of the medical scheme (Figure 1): Figure 1: General anatomy of the human brain Marieb & Hoehn (Human Anatomy and Physiology, 9th ed.) – Figure 12.2 CEREBRUM: Divided into two hemispheres, the cerebrum is the largest region of the human brain – the two hemispheres together account for ~ 85% of total brain mass. The cerebrum forms the superior part of the brain, covering and obscuring the diencephalon and brain stem similar to the way a mushroom cap covers the top of its stalk. Elevated ridges of tissue, called gyri (singular: gyrus), separated by shallow groves called sulci (singular: sulcus) mark nearly the entire surface of the cerebral hemispheres. Deeper groves, called fissures, separate large regions of the brain. Much of the cerebrum is involved in the processing of somatic sensory and motor information as well as all conscious thoughts and intellectual functions. The outer cortex of the cerebrum is composed of gray matter – billions of neuron cell bodies and unmyelinated axons arranged in six discrete layers. Although only 2 – 4 mm thick, this region accounts for ~ 40% of total brain mass. The inner region is composed of white matter – tracts of myelinated axons. -
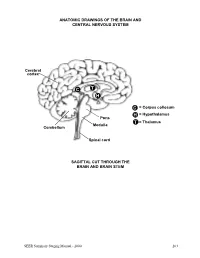
Brain and Central Nervous System
ANATOMIC DRAWINGS OF THE BRAIN AND CENTRAL NERVOUS SYSTEM Cerebral cortex C T H C = Corpus collosum H = Hypothalamus Pons T = Thalamus Medulla Cerebellum Spinal cord SAGITTAL CUT THROUGH THE BRAIN AND BRAIN STEM SEER Summary Staging Manual - 2000 263 ANATOMIC DRAWINGS OF THE BRAIN AND CENTRAL NERVOUS SYSTEM 2 1 3 4 7 5 8 6 SAGITTAL CUT THROUGH THE HUMAN HEAD WITH CEREBRUM IN PLACE The cerebrum is comprised of the: 1 Frontal lobe 2 Parietal lobe 3 Temporal lobe 4 Occipital lobe Other parts of the brain include: 5 Pons 6 Medulla (oblongata) 7 Cerebellum 8 Tentorium (cerebelli) 264 SEER Summary Staging Manual - 2000 ANATOMIC DRAWINGS OF THE BRAIN AND CENTRAL NERVOUS SYSTEM A B C D E 7 5 6 8 F SAGITTAL CUT THROUGH THE HUMAN HEAD Internal anatomy of the brain: A Inner surface of right hemisphere of cerebrum B Corpus callosum C Velum interpositum D Middle commissure E Third ventricle F Fourth ventricle Other parts of the brain (as on previous drawing): 5 Pons 6 Medulla (oblongata) 7 Cerebellum 8 Tentorium (cerebelli) SEER Summary Staging Manual - 2000 265 BRAIN AND CEREBRAL MENINGES C70.0, C71.0-C71.9 Supratentorial (S) or Infratentorial (I) C70.0 Cerebral meninges C71.0 Cerebrum ? (S) C71.1 Frontal lobe (S) C71.2 Temporal lobe (S) C71.3 Parietal lobe (S) C71.4 Occipital lobe (S) C71.5 Ventricle, NOS (S) C71.6 Cerebellum, NOS (I) C71.7 Brain stem (I) C71.8 Overlapping lesion of brain ? C71.9 Brain, NOS ? ?See Note 1. SUMMARY STAGE 1 Localized only Supratentorial tumor confined to: Cerebral hemisphere (cerebrum) or meninges of cerebral hemisphere -

High Five for Safe Arterial Blood Gas Sampling
High Five for safe arterial blood gas sampling 1. Carraro P et al. Errors in a stat laboratory: Types and frequencies 10 years later. Clin Chem 2007; 53,7: 1338-42. Agenda . Introduction . Why the preanalytical phase is important . High Five for safe arterial blood gas sampling . Additional educational resources The preanalytical phase of arterial blood gas sampling Preanalytical errors are said “Several aspects of blood pH and to be the reason for up to gas analysis are unique among 62% of all errors in clinical and laboratory determinations, and, at the same laboratory medicine [1]. time, no other test results have more immediate impact on Error rate patient care” [2] Preanalytical phase 62% CLSI Analytical phase 15% Post-analytical phase 23% 1. Carraro P et al. Errors in a stat laboratory: Types and frequencies 10 years later. Clin Chem 2007; 53,7: 1338-42. 2. CLSI. Blood Gas and pH Analysis and Related Measurements; Approved Guideline – Second Edition. CLSI Document C46-A2. Wayne, PA: Clinical and Laboratory Standards Institute: 2009. 3. www.clsi.org. Safe arterial blood gas sampling: Path of workflow [1,2]: 1. Patient preparation – FOCUS ON: patient safety 2. Blood collection device FOCUS ON: sample integrity and operator safety 3. Sample collection FOCUS ON: safety for patient and operator 4. Sample handling FOCUS ON: sample integrity 5. Sample transport FOCUS ON: time to patient results 1. Clinical and Laboratory Standards Institute (CLSI). Procedures for the Collection of Arterial Blood Specimens; Approved Standard-Fourth Edition. H11-A4. Vol. 24 No. 28 [ISBN 1-56238-427-9]. Clinical and Laboratory Standards Institute, 940 West Valley Road, Suite 1400, Wayne, Pennsylvania 19087-1898, USA. -

Review of the Nomenclature of the Liver Anatomical and Functional Areas by Three-Dimensional Volume Rendering 64-Multislice Computed Tomography
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Firenze University Press: E-Journals IJAE Vol. 119, n. 3: 169-179, 2014 ITALIAN JOURNAL OF ANATOMY AND EMBRYOLOGY Research Article – Basic and Applied Anatomy Review of the nomenclature of the liver anatomical and functional areas by three-dimensional volume rendering 64-multislice computed tomography. Proposal for an update of the terminology Sergio Castorina1,2* 1 Department of Bio-Medical Sciences, University of Catania, 95125 Catania, Italy 2 Fondazione Mediterranea “G.B. Morgagni”, 95125 Catania, Italy Submitted September 13, 2013; accepted revised December 13, 2013 Abstract In the last centuries, the anatomy of the liver has been the object of increasing interest. The Inter- national Anatomical Terminology tries to unify the terminology of liver anatomy, making it a liv- ing language. A single, worldwide-accepted classification of the liver still does not exist. In fact, definition of segments according to Couinaud’s nomenclature is different from that of Goldsmith and Woodburne. The aim of this paper was to revise the liver topography by 64-Multislice Com- puted Tomography, in patients who had undergone repair of cholelithiasis, starting from classifi- cations based on the efferent venous system or on the Glissonian system. This technique allows to remove virtually the liver parenchyma, and, together with the subsequent three-dimensional reconstruction of images, represents the best tool to visualise the hepatic ducts and segments. Through this approach, we propose a new terminology, which considers the liver divided into five lobes and seven segments plus one caudate lobe. -

Phlebotomy Guidelines and Order of Draw
165 Ashley Ave., Room 318 Charleston, SC 29425 Phone (843) 792-0707 Fax (843) 792-4896 Phlebotomy Guidelines and Order of Draw Specimen Collection Procedures The purpose of the document is to share the standard criteria for venous blood collection for medical laboratory testing. Proper specimen collection and handling is a critical part of obtaining a valid laboratory result. Specimens must be collected in the appropriate collection container, kit or device, correctly labeled and transported promptly to the laboratory. Staff responsible for sample collection should follow essential safeguards to ensure accurate testing and to provide quality patient care. 1. Always check patient identification band and compare to the name on the requisition and the specimen labels. Label specimens immediately following collection. Labels must be rechecked before sending to the lab. 2. Deliver specimens to the laboratory as soon after collection as possible. Certain analytes are unstable and testing should occur as soon after collection as possible to insure valid measurements. 3. Special Collection Procedures: o When obtaining a blood specimen from a catheter, the components of the blood collection system (catheter, luer lock, syringe, needle, and collection device) should be checked to ensure compatibility to avoid air leaks which may cause hemolysis and incorrect draw volumes. o Collection of the blood through lines that have been previously flushed with heparin should be avoided, if possible. o If the blood must be drawn through an indwelling catheter, possible heparin contamination and specimen dilution should be considered. The line should be flushed with 5 ml of saline and the first 5 ml of blood or six dead space volumes of the catheter discarded.