Systematics and Phylogeography of the Mediterranean Helichrysum
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(Ou Immortelles) En Aromathérapie: Zoom Sur L'hélichryse Italienne Corse
Les hélichryses (ou immortelles) en aromathérapie : zoom sur l’Hélichryse italienne corse Margaux Degrelle To cite this version: Margaux Degrelle. Les hélichryses (ou immortelles) en aromathérapie : zoom sur l’Hélichryse italienne corse. Sciences pharmaceutiques. 2015. hal-01731734 HAL Id: hal-01731734 https://hal.univ-lorraine.fr/hal-01731734 Submitted on 14 Mar 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. AVERTISSEMENT Ce document est le fruit d'un long travail approuvé par le jury de soutenance et mis à disposition de l'ensemble de la communauté universitaire élargie. Il est soumis à la propriété intellectuelle de l'auteur. Ceci implique une obligation de citation et de référencement lors de l’utilisation de ce document. D'autre part, toute contrefaçon, plagiat, reproduction illicite encourt une poursuite pénale. Contact : [email protected] LIENS Code de la Propriété Intellectuelle. articles L 122. 4 Code de la Propriété Intellectuelle. articles L 335.2- L 335.10 http://www.cfcopies.com/V2/leg/leg_droi.php http://www.culture.gouv.fr/culture/infos-pratiques/droits/protection.htm -

1965, Five Just As in Robert Frost's, "The Road Little Skiing When He Can
KNIGHT BEACON BoostersBring College To Nigl,School We the students of Assumption High resentative to start his presentation at St. Mary's College, Winona, Minnesota; Soon to college must apply a c rtain time for one group of people. and St. Tho mas College, St. Paul, Min We know not where, or how, or when, Fr. Charles Mann, boys' division vice nesota. But that' where College ight comes principal noted, "The system worked Refreshments will be served in the in! well for the colleges that used it last cafeteria during the evening. This year on Wednesday, October y ar, and we hope it will work again 15, at 7:30 Assumption high school's this year." annual College Night will take place . Three new addition are fore. een in A coll ge atmosphere will be enacted this year' chedule. Tho e hool are: when over 40 colleges, universities, The College of t. Benedict, t. Joseph, Knite technical colleges, and nursing colleges linnesota, Loras College, Dubuque, will send representatives to the event. Iowa, and Edgewood College of the acred Heart, Madison, Wi consin. Lite Being ponsored by the Booster Club Besides Marycrest and St. Ambrose, again thi year, a rewarding night is in to which most AHS graduates apply, store for everyone. ophomore , jun ther will be other schools which have I'll bet everyone's eyes were on Sr . iors, and eniors are invited to come, participated in College Night before . Mary Ambrosina, BVM, when she compare, and judge the college so Among these are: John Carroll Univer said, "If you'll pay attention, I'll go that they can make a good decision on sity, Cleveland, Ohio; Western Illinois through the board." a pecific college. -
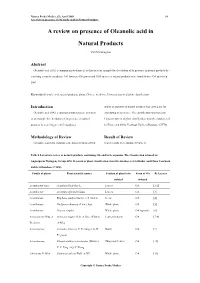
A Review on Presence of Oleanolic Acid in Natural Products
Natura Proda Medica, (2), April 2009 64 A review on presence of Oleanolic acid in Natural Products A review on presence of Oleanolic acid in Natural Products YEUNG Ming Fai Abstract Oleanolic acid (OA), a common phytochemical, is chosen as an example for elucidation of its presence in natural products by searching scientific databases. 146 families, 698 genera and 1620 species of natural products were found to have OA up to Sep 2007. Keywords Oleanolic acid, natural products, plants, Chinese medicine, Linnaeus system of plant classification Introduction and/or its saponins in natural products was carried out for Oleanolic acid (OA), a common phytochemical, is chosen elucidating its pressence. The classification was based on as an example for elucidation of its presence in natural Linnaeus system of plant classification from the databases of products by searching scientific databases. SciFinder and China Yearbook Full-text Database (CJFD). Methodology of Review Result of Review Literature search for isolation and characterization of OA Search results were tabulated (Table 1). Table 1 Literature review of natural products containing OA and/or its saponins. The classification is based on Angiosperm Phylogeny Group APG II system of plant classification from the databases of SciFinder and China Yearbook Full-text Database (CJFD). Family of plants Plant scientific names Position of plant to be Form of OA References isolated isolated Acanthaceae Juss. Acanthus illicifolius L. Leaves OA [1-2] Acanthaceae Avicennia officinalis Linn. Leaves OA [3] Acanthaceae Blepharis sindica Stocks ex T. Anders Seeds OA [4] Acanthaceae Dicliptera chinensis (Linn.) Juss. Whole plant OA [5] Acanthaceae Justicia simplex Whole plant OA saponins [6] Actinidiaceae Gilg. -

Listado De Todas Las Plantas Que Tengo Fotografiadas Ordenado Por Familias Según El Sistema APG III (Última Actualización: 2 De Septiembre De 2021)
Listado de todas las plantas que tengo fotografiadas ordenado por familias según el sistema APG III (última actualización: 2 de Septiembre de 2021) GÉNERO Y ESPECIE FAMILIA SUBFAMILIA GÉNERO Y ESPECIE FAMILIA SUBFAMILIA Acanthus hungaricus Acanthaceae Acanthoideae Metarungia longistrobus Acanthaceae Acanthoideae Acanthus mollis Acanthaceae Acanthoideae Odontonema callistachyum Acanthaceae Acanthoideae Acanthus spinosus Acanthaceae Acanthoideae Odontonema cuspidatum Acanthaceae Acanthoideae Aphelandra flava Acanthaceae Acanthoideae Odontonema tubaeforme Acanthaceae Acanthoideae Aphelandra sinclairiana Acanthaceae Acanthoideae Pachystachys lutea Acanthaceae Acanthoideae Aphelandra squarrosa Acanthaceae Acanthoideae Pachystachys spicata Acanthaceae Acanthoideae Asystasia gangetica Acanthaceae Acanthoideae Peristrophe speciosa Acanthaceae Acanthoideae Barleria cristata Acanthaceae Acanthoideae Phaulopsis pulchella Acanthaceae Acanthoideae Barleria obtusa Acanthaceae Acanthoideae Pseuderanthemum carruthersii ‘Rubrum’ Acanthaceae Acanthoideae Barleria repens Acanthaceae Acanthoideae Pseuderanthemum carruthersii var. atropurpureum Acanthaceae Acanthoideae Brillantaisia lamium Acanthaceae Acanthoideae Pseuderanthemum carruthersii var. reticulatum Acanthaceae Acanthoideae Brillantaisia owariensis Acanthaceae Acanthoideae Pseuderanthemum laxiflorum Acanthaceae Acanthoideae Brillantaisia ulugurica Acanthaceae Acanthoideae Pseuderanthemum laxiflorum ‘Purple Dazzler’ Acanthaceae Acanthoideae Crossandra infundibuliformis Acanthaceae Acanthoideae Ruellia -

Monitoring of Alien Aquatic Plants in the Inland Waters of Sicily (Italy) Citation: Troia A
Journal of Plant Firenze University Press Taxonomy www.fupress.com/webbia WEBBIA and Geography Monitoring of alien aquatic plants in the inland waters of Sicily (Italy) Citation: Troia A. et al. (2020) Monitor- ing of alien aquatic plants in the inland waters of Sicily (Italy). Webbia. Jour- nal of Plant Taxonomy and Geography Angelo Troia1,*, Vincenzo Ilardi2, Elisabetta Oddo1 75(1): 77-83. doi: 10.36253/jopt-8414 1 Dipartimento STEBICEF (Scienze e Tecnologie Biologiche, Chimiche e Farmaceutiche), Received: April 2, 2020 Università degli Studi di Palermo, Palermo, Italy 2 Dipartimento DISTEM (Scienze della Terra e del Mare), Università degli Studi di Paler- Accepted: May 8, 2020 mo, Palermo, Italy Published: June 30, 2020 *Corresponding author, email [email protected] Copyright: © 2020 A. Troia, V. Ilardi, E. Oddo. This is an open access, peer- Abstract. Updated and reliable data on the presence and distribution of alien aquatic reviewed article published by Firenze plant species in Sicily are lacking, and there is a need to fill this gap for a proper and University Press (http://www.fupress. efficient management of freshwater ecosystems and biodiversity. This paper reviews com/webbia) and distributed under the the available knowledge about alien aquatic vascular plants in the inland waters of terms of the Creative Commons Attri- Sicily (Italy). The aim is to provide an updated checklist, as a first step in the study of bution License, which permits unre- the impact of those plants on the native species and ecosystems of this Mediterranean stricted use, distribution, and reproduc- island. The paper focuses on the strictly aquatic species (hydrophytes), excluding emer- tion in any medium, provided the origi- gent macrophytes. -
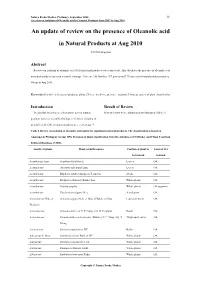
An Update of Review on the Presence of Oleanolic Acid in Natural Products at Aug 2010
Natura Proda Medica, Prelimary, September 2010 11 A review on isolation of Oleanolic acid in Natural Products from 2007 to Aug 2010 An update of review on the presence of Oleanolic acid in Natural Products at Aug 2010 YEUNG Ming Fai Abstract Reviews on isolation of oleanolic acid (OA) in natural products were carried out. This elucidates the presence of oleanolic acid in natural products based on scientific findings. There are 158 families, 767 genera and 1710 species of natural products isolated OA up to Aug 2010. Keywords Oleanolic acid, natural products, plants, Chinese medicine, presence, isolation, Linnaeus system of plant classification Introduction Result of Review To elucidate the presence of oleanolic acid in natural Review results were collaborated and tabulated (Table 1). products based on scientific findings, reviews on isolation of oleanolic acid (OA) in natural products were carried out 1-2. Table 1 Review on isolation of oleanolic acid and/or its saponins in natural products. The classification is based on Angiosperm Phylogeny Group APG II system of plant classification from the databases of SciFinder and China Yearbook Full-text Database (CJFD). Family of plants Plant scientific names Position of plant to Form of OA be isolated isolated Acanthaceae Juss. Acanthus illicifolius L. Leaves OA Acanthaceae Avicennia officinalis Linn. Leaves OA Acanthaceae Blepharis sindica Stocks ex T. Anders Seeds OA Acanthaceae Dicliptera chinensis (Linn.) Juss. Whole plants OA Acanthaceae Justicia simplex Wholeplants OAsaponins Acanthaceae Gendarussa vulgaris Nees Aerial parts OA Actinidiaceae Gilg. et Actinidia arguta (Sieb. et Zucc.) Planch. ex Miq. Leaves or stems OA Werderm. Actinidiaceae Actinidia deliciosa C. -

Caractérisation Par CPG/IK, CPG/SM Et RMN Du Carbone-13 D'huiles
Caractérisation par CPG/IK, CPG/SM et RMN du carbone-13 d’huiles essentielles de Madagascar Jean-François Cavalli To cite this version: Jean-François Cavalli. Caractérisation par CPG/IK, CPG/SM et RMN du carbone-13 d’huiles essen- tielles de Madagascar. Autre. Université Pascal Paoli, 2002. Français. tel-00007939 HAL Id: tel-00007939 https://tel.archives-ouvertes.fr/tel-00007939 Submitted on 7 Jan 2005 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. UNIVERSITE DE CORSE PASCAL PAOLI FACULTE DES SCIENCES ET TECHNIQUES THESE pour obtenir le grade de DOCTEUR DE L’UNIVERSITE DE CORSE Discipline : Chimie Organique et Analytique présentée et soutenue publiquement par Jean-François CAVALLI le 17-10-2002 CARACTERISATION PAR CPG/IK, CPG/SM ET RMN DU CARBONE-13 D’HUILES ESSENTIELLES DE MADAGASCAR Directeurs de thèse : Pr. Antoine-François BERNARDINI Pr. Joseph CASANOVA JURY Mme L. LIZZANI-CUVELIER Professeur, Université de Nice Sophia-Antipolis (Rapporteur) M. E. GAYDOU Professeur, Université de Marseille (Rapporteur) M. A.-F. BERNARDINI Professeur, Université de Corse M. J. CASANOVA Professeur, Université de Corse M. F. TOMI Professeur, Université de Corse A A mes parents, A ma sœur. -

Genetic Diversity and Local Population Structure in Ambrosina Bassii (Araceae, Ambrosineae), a Mediterranean Relict Species
Biochemical Systematics and Ecology 37 (2009) 737–746 Contents lists available at ScienceDirect Biochemical Systematics and Ecology journal homepage: www.elsevier.com/locate/biochemsyseco Genetic diversity and local population structure in Ambrosina bassii (Araceae, Ambrosineae), a Mediterranean relict species Anna Geraci*, Francesco Maria Raimondo, Angelo Troia Dipartimento di Scienze Botaniche, Universita` degli Studi di Palermo, via Archirafi 28, I-90123 Palermo, Italy article info abstract Article history: The effects of habitat fragmentation on the genetic structure of Ambrosina bassii are Received 2 April 2009 analyzed. The species, whose reproductive biology is mostly unknown, is the only repre- Accepted 5 December 2009 sentative of its genus and tribe and it is endemic to the central Mediterranean area. The selected study area was the island of Sicily, in which wild populations show a wide Keywords: morphological variability and ecological amplitude. Patterns of within- and among-pop- Ambrosina bassii ulation genetic diversity in eleven Sicilian populations, occurring in six disjunct areas, Araceae were examined by means of allozyme electrophoresis. High levels of genetic diversity were Island Sicily found as shown by the mean expected heterozygosity (He ¼ 0.263), the percentage of Fragmentation polymorphic loci (P95 ¼ 65.3), the mean number of alleles per locus (A ¼ 2.0). Genetic Disjunct populations differentiation between populations was relatively low (mean FST ¼ 0.091 and Nm ¼ 1.98). Genetic structure A very weak correlation exists between genetic distances and geographic distances Allozymes between populations. Despite its restricted and fragmented geographical range, A. bassii showed (i) high levels of genetic diversity, mainly within populations; (ii) no genetic differentiation between populations and morphotypes. -

Aim of the Conference
/RIGINAND%VOLUTIONOF"IOTA IN-EDITERRANEAN#LIMATE:ONES AN)NTEGRATIVE6ISION /RGANIZINGCOMMITTEE %LENA#ONTI #HAIR #ORINNE"URLET !LESSIA'UGGISBERG "ARBARA+ELLER 'UILHEM-ANSION 'ABRIELE3ALVO *ULY )NSTITUTEOF3YSTEMATIC"OTANY 5NIVERSITYOF:URICH 3PONSORS +PUVKVWVGQH5[UVGOCVKE$QVCP[ &GCPQH5EKGPEG1HHKEG 7PKXGTUKV[QH<WTKEJ Aim of the conference Understanding the biotic and abiotic processes that contribute to high species numbers in biodiversity 'hot spots' is one of the major tasks of biology. The exceptional biological richness of the five mediterranean climate zones of the earth - the Mediterranean basin, South Africa, Australia, Chile and California - makes them an ideal case study to investigate the evolutionary and ecological dynamics that generate elevated species numbers. By focusing on the Mediterranean basin, the conference will synthesize the current state of knowledge on the origin of mediterranean biota, while charting a map for pushing the frontier of conceptual and methodological advances in biodiversity studies. The goal of the conference is to clarify the history of biotic assembly in mediterranean climate zones by integrating evidence across multiple disciplines, including evolutionary biology, systematics, ecology, paleontology, paleoclimatology, and paleogeology. The conference is aimed at scholars from various biodiversity disciplines at different stages of their careers, from beginning Ph.D. students to established scholars. Marmaris, Turkey, June 2005 (Photo: G. Mansion) Organization committee Prof. Elena Conti Chair -

W. Greuter & G. Domina
Bocconea 27(1): 21-61 doi: 10.7320/Bocc27.1.021 Version of Record published online on 17 December 2015 Werner Greuter & Gianniantonio Domina Checklist of the vascular plants collected during the 12th “Iter Mediterraneum” in Tunisia, 24 March – 4 April 2014 Abstract Greuter, W. & Domina, G.: Checklist of the vascular plants collected during the 12th “Iter Mediterraneum” in Tunisia, 24 March – 4 April 2014. — Bocconea 27(1): 21-61. 2015. — ISSN: 1120-4060 printed, 2280-3882 online. The vascular plants (plus one alga) collected during Iter Mediterraneum XII of OPTIMA in North Tunisia have been studied. In all, 1374 gatherings were made from 43 localities in the “Cap Bon” region, “Dorsale Tunisienne”, “Tunisie du Nord-Est”, “Vallée de la Medjedah”, “Mogods” and “Kroumirie” areas. They belong to 82 families and 539 species or subspecies. Three taxa are new for the flora of Tunisia (Cerastium diffusum subsp. gussonei, Senecio leucanthemifolius subsp. mauritanicus, and Ulmus minor subsp. canescens), 7 represent new records for the “Kroumirie”, 15 for the “Mogods”, 3 for the “Tunisie du Nord-Est”, 2 for the “Dorsale Tunisienne”, and 8 for “Cap Bon”. Key words: Flora of Tunisia, Kroumiria, Cape Bon, Itinera Mediterranea, OPTIMA, vascular plants. Collecting localities A list of collecting localities, drawn up immediately and daily updated as the excursion progressed, was used as basis for labelling. The locality data were checked and confirmed, as to accuracy of place names and consistency of spelling, by our Tunesian hosts, in par- ticular Ridha El Mokni. The coordinates were ascertained on the spot by means of the global positioning system (GPS) Garmin GPS map CX60. -

Complete List of Literature Cited* Compiled by Franz Stadler
AppendixE Complete list of literature cited* Compiled by Franz Stadler Aa, A.J. van der 1859. Francq Van Berkhey (Johanes Le). Pp. Proceedings of the National Academy of Sciences of the United States 194–201 in: Biographisch Woordenboek der Nederlanden, vol. 6. of America 100: 4649–4654. Van Brederode, Haarlem. Adams, K.L. & Wendel, J.F. 2005. Polyploidy and genome Abdel Aal, M., Bohlmann, F., Sarg, T., El-Domiaty, M. & evolution in plants. Current Opinion in Plant Biology 8: 135– Nordenstam, B. 1988. Oplopane derivatives from Acrisione 141. denticulata. Phytochemistry 27: 2599–2602. Adanson, M. 1757. Histoire naturelle du Sénégal. Bauche, Paris. Abegaz, B.M., Keige, A.W., Diaz, J.D. & Herz, W. 1994. Adanson, M. 1763. Familles des Plantes. Vincent, Paris. Sesquiterpene lactones and other constituents of Vernonia spe- Adeboye, O.D., Ajayi, S.A., Baidu-Forson, J.J. & Opabode, cies from Ethiopia. Phytochemistry 37: 191–196. J.T. 2005. Seed constraint to cultivation and productivity of Abosi, A.O. & Raseroka, B.H. 2003. In vivo antimalarial ac- African indigenous leaf vegetables. African Journal of Bio tech- tivity of Vernonia amygdalina. British Journal of Biomedical Science nology 4: 1480–1484. 60: 89–91. Adylov, T.A. & Zuckerwanik, T.I. (eds.). 1993. Opredelitel Abrahamson, W.G., Blair, C.P., Eubanks, M.D. & More- rasteniy Srednei Azii, vol. 10. Conspectus fl orae Asiae Mediae, vol. head, S.A. 2003. Sequential radiation of unrelated organ- 10. Isdatelstvo Fan Respubliki Uzbekistan, Tashkent. isms: the gall fl y Eurosta solidaginis and the tumbling fl ower Afolayan, A.J. 2003. Extracts from the shoots of Arctotis arcto- beetle Mordellistena convicta. -

Pollinators and Visitors of Aroid Inflorescences
Pollinators and Visitors of Aroid Inflorescences Marc Gibernau Laboratoire d’E´volution & Diversite´Biologique Universite´de Toulouse III 118 Route de Narbonne, Baˆt. IV R 3—B 2 31062 Toulouse Cedex 4 France e-mail: [email protected] ABSTRACT view of this subject, as Thomas Croat (2000) did in his history and current status Data on aroid pollinators was first sum- of systematic research with Araceae, but to marized by Grayum (1984) who docu- give, in the first place, a statement of the mented 35 genera and about 90 species. A subject and to develop some remarks on second summary was published in 1997 in aroid pollination. The Genera of Araceae (Mayo et al., 1997) with 38 genera and less than 100 species listed including data from Grayum (1986, RESULTS 1990). This paper brings the reference list Summarizing data from Grayum (1984, up to date since 1997, documenting the 1990) and Mayo et al. (1997), and includ- pollinators of 49 genera and about 125 ing omitted and new publications, the pol- species. These numbers are still very low in comparison with the diversity of the Ar- linators of 49 genera and about 125 spe- aceae family which contains 105 genera cies are documented in Table 1. These and about 3,300 species. Some questions numbers are still very low in comparison on aroid pollination are developed in the with the diversity of the Araceae family discussion. which contains 105 genera and about 3,300 species (Mayo et al., 1997). Thus, KEY WORDS pollinators are cited for only 47% of the genera.