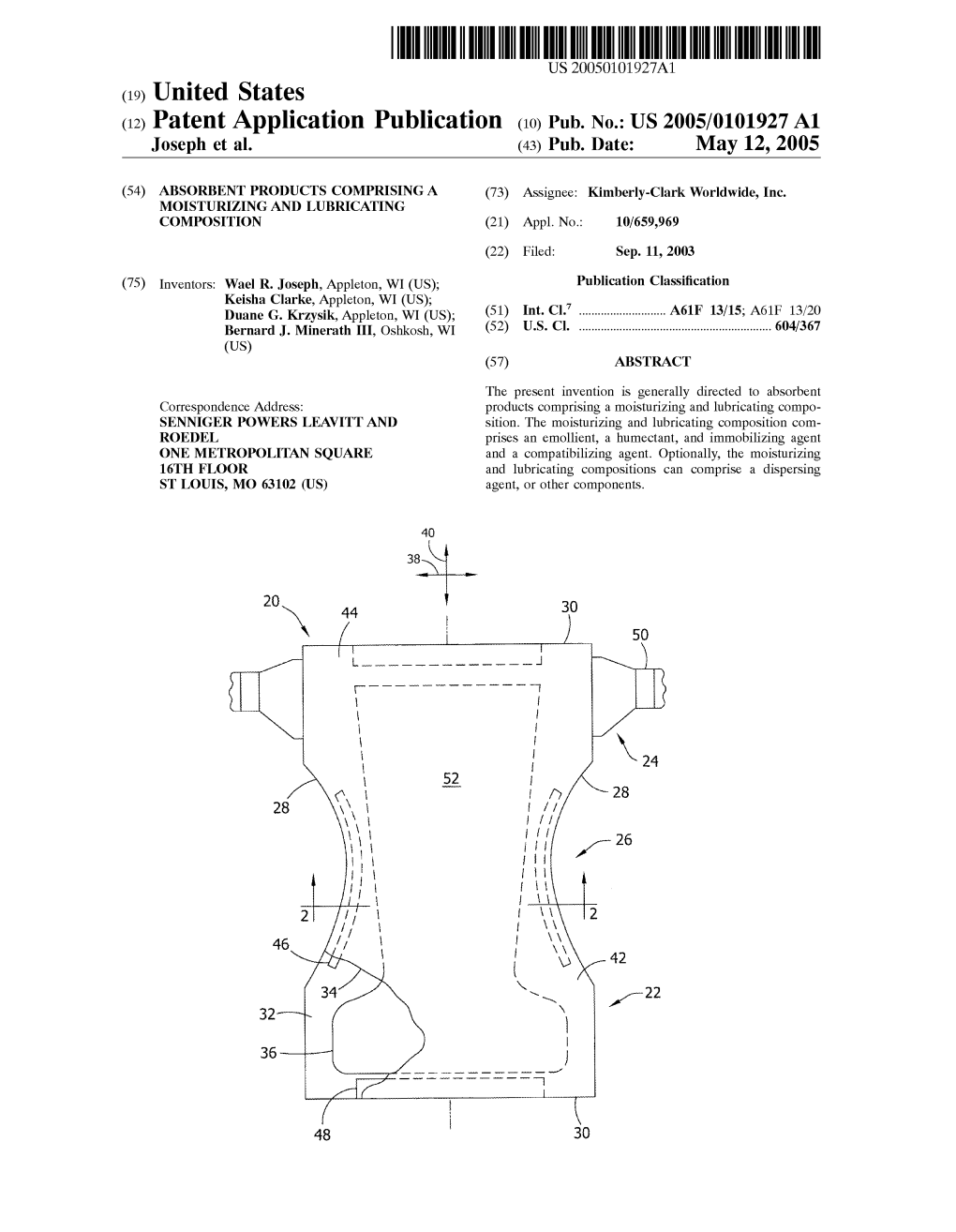
(12) Patent Application Publication (10) Pub. No.: US 2005/0101927 A1 Joseph Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

• Our Bodies Make All the Cholesterol We Need. • 85 % of Our Blood
• Our bodies make all the cholesterol we need. • 85 % of our blood cholesterol level is endogenous • 15 % = dietary from meat, poultry, fish, seafood and dairy products. • It's possible for some people to eat foods high in cholesterol and still have low blood cholesterol levels. • Likewise, it's possible to eat foods low in cholesterol and have a high blood cholesterol level SYNTHESIS OF CHOLESTEROL • LOCATION • All tissues • Liver • Cortex of adrenal gland • Gonads • Smooth endoplasmic reticulum Cholesterol biosynthesis and degradation • Diet: only found in animal fat • Biosynthesis: primarily synthesized in the liver from acetyl-coA; biosynthesis is inhibited by LDL uptake • Degradation: only occurs in the liver • Cholesterol is only synthesized by animals • Although de novo synthesis of cholesterol occurs in/ by almost all tissues in humans, the capacity is greatest in liver, intestine, adrenal cortex, and reproductive tissues, including ovaries, testes, and placenta. • Most de novo synthesis occurs in the liver, where cholesterol is synthesized from acetyl-CoA in the cytoplasm. • Biosynthesis in the liver accounts for approximately 10%, and in the intestines approximately 15%, of the amount produced each day. • Since cholesterol is not synthesized in plants; vegetables & fruits play a major role in low cholesterol diets. • As previously mentioned, cholesterol biosynthesis is necessary for membrane synthesis, and as a precursor for steroid synthesis including steroid hormone and vitamin D production, and bile acid synthesis, in the liver. • Slightly less than half of the cholesterol in the body derives from biosynthesis de novo. • Most cells derive their cholesterol from LDL or HDL, but some cholesterol may be synthesize: de novo. -

01 Excipients Prelims 1..9
Triolein 757 and tablets). Included in the Canadian List of Acceptable Non- 3 Steurnagel CR. Latex emulsions for controlled drug delivery. McGinity medicinal Ingredients. JW, ed. Aqueous Polymeric Coatings for Pharmaceutical Dosage Forms. New York: Marcel Dekker, 1989; 1–61. 4 Gutierrez-Rocca JC, McGinity JW. Influence of aging on the physical– 17 Related Substances mechanical properties of acrylic resin films cast from aqueous Acetyltributyl citrate; acetyltriethyl citrate; tributyl citrate. dispersions and organic solutions. Drug Dev Ind Pharm 1993; 19(3): 315–332. 5 Liu J, Williams R. Properties of heat-humidity cured cellulose acetate 18 Comments phthalate free films. Eur J Pharm Sci 2002; 17(1–2): 31–41. 6 Lewis RJ, ed. Sax’s Dangerous Properties of Industrial Materials, 11th A specification for triethyl citrate is contained in the Food (7) edn. New York: Wiley, 2004; 3546. Chemicals Codex (FCC). 7 Food Chemicals Codex, 6th edn. Bethesda, MD: United States The EINECS number for triethyl citrate is 201-070-7. The Pharmacopeia, 2008; 988. PubChem Compound ID (CID) for triethyl citrate is 6506. 20 General References 19 Specific References Vertellus Specialties Inc. Technical data sheet: Citroflex 2, 2007. 1 Gutierrez-Rocca JC, McGinity JW. Influence of water soluble and insoluble plasticizers on the physical and mechanical properties of 21 Author acrylic resin copolymers. Int J Pharm 1994; 103: 293–301. J Teckoe. 2 Lehmann K. Chemistry and application properties of polymethacrylate coating systems. McGinity JW, ed. Aqueous Polymeric Coatings for Pharmaceutical Dosage Forms. New York: Marcel Dekker, 1989; 153– 22 Date of Revision 245. 24 February 2009. Triolein 1 Nonproprietary Names 6 Functional Category None adopted. -

Intravenous Treatment with a Long-Chain Omega-3 Lipid Emulsion Provides Neuroprotection in a Murine Model of Ischemic Stroke – a Pilot Study
RESEARCH ARTICLE Intravenous Treatment with a Long-Chain Omega-3 Lipid Emulsion Provides Neuroprotection in a Murine Model of Ischemic Stroke ± A Pilot Study Dirk Berressem1*, Konrad Koch1, Nicole Franke1, Jochen Klein1, Gunter P. Eckert1,2 1 Goethe-University of Frankfurt, Department of Pharmacology, Germany, 2 Justus-Liebig-University Giessen, Institute of Nutritional Sciences, Germany * [email protected] a11111 Abstract Single long-chain omega-3 fatty acids (e.g. docosahexaenoic acid (DHA) or eicosapentae- noic acid (EPA)) are known for their neuroprotective properties associated with ischemic stroke. This pilot study aimed to test the effectiveness of an acute treatment with a long- OPEN ACCESS chain omega-3 lipid emulsion (Omegaven 10%®, OGV) that contains fish oil (DHA 18 mg/ Citation: Berressem D, Koch K, Franke N, Klein J, Eckert GP (2016) Intravenous Treatment with a ml; EPA 21 mg/ml) and α-tocopherol (0.2 mg/ml) in a transient middle cerebral artery occlu- Long-Chain Omega-3 Lipid Emulsion Provides sion (MCAO) model of ischemic stroke in mice. For this purpose, female CD-1 mice were Neuroprotection in a Murine Model of Ischemic anesthetized and subjected to 90 minutes of MCAO. To reflect a clinically relevant situation Stroke ± A Pilot Study. PLoS ONE 11(11): for an acute treatment, either after induction of stroke or after reperfusion, a single dose of e0167329. doi:10.1371/journal.pone.0167329 OGV was injected intravenously into the tail vein (5 ml/kg b.w.). A neurological severity Editor: Muzamil Ahmad, Indian Institute of score was used to assess motor function and neurological outcome. -

33 34 35 Lipid Synthesis Laptop
BI/CH 422/622 Liver cytosol ANABOLISM OUTLINE: Photosynthesis Carbohydrate Biosynthesis in Animals Biosynthesis of Fatty Acids and Lipids Fatty Acids Triacylglycerides contrasts Membrane lipids location & transport Glycerophospholipids Synthesis Sphingolipids acetyl-CoA carboxylase Isoprene lipids: fatty acid synthase Ketone Bodies ACP priming 4 steps Cholesterol Control of fatty acid metabolism isoprene synth. ACC Joining Reciprocal control of b-ox Cholesterol Synth. Diversification of fatty acids Fates Eicosanoids Cholesterol esters Bile acids Prostaglandins,Thromboxanes, Steroid Hormones and Leukotrienes Metabolism & transport Control ANABOLISM II: Biosynthesis of Fatty Acids & Lipids Lipid Fat Biosynthesis Catabolism Fatty Acid Fatty Acid Synthesis Degradation Ketone body Utilization Isoprene Biosynthesis 1 Cholesterol and Steroid Biosynthesis mevalonate kinase Mevalonate to Activated Isoprenes • Two phosphates are transferred stepwise from ATP to mevalonate. • A third phosphate from ATP is added at the hydroxyl, followed by decarboxylation and elimination catalyzed by pyrophospho- mevalonate decarboxylase creates a pyrophosphorylated 5-C product: D3-isopentyl pyrophosphate (IPP) (isoprene). • Isomerization to a second isoprene dimethylallylpyrophosphate (DMAPP) gives two activated isoprene IPP compounds that act as precursors for D3-isopentyl pyrophosphate Isopentyl-D-pyrophosphate all of the other lipids in this class isomerase DMAPP Cholesterol and Steroid Biosynthesis mevalonate kinase Mevalonate to Activated Isoprenes • Two phosphates -

Steroid Interference with Antifungal Activity of Polyene Antibiotics
APPLIED MICROBIOLOGY, Nov., 1966 Vol. 14, No. 6 Copyright © 1966 American Society for Microbiology Printed in U.S.A. Steroid Interference with Antifungal Activity of Polyene Antibiotics WALTER A. ZYGMUNT AND PETER A. TAVORMINA Department of Microbiology and Natural Products Research, Mead Johnson & Company, Evansville, Indiana Received for publication 21 April 1966 ABSTRACT ZYGMUNT, WALTER A. (Mead Johnson & Co., Evansville, Ind.), AND PETER A. TAVORMINA. Steroid interference with antifungal activity of polyene antibiotics. Appl. Microbiol. 14:865-869. 1966.-Wide differences exist among the polyene antibiotics, nystatin, rimocidin, filipin, pimaricin, and amphotericin B, with ref- erence to steroid interference with their antifungal activities against Candida albicans. Of the numerous steroids tested, ergosterol was the only one which ef- fectively antagonized the antifungal activity of all five polyene antibiotics. The antifungal activities of nystatin and amphotericin B were the least subject to vitia- tion by the addition of steroids other than ergosterol, and those of filipin, rimo- cidin, and pimaricin were the most sensitive to interference. Attempts to delineate the structural requirements of steroids possessing polyene-neutralizing activity in growing cultures of C. albicans are discussed. The ultraviolet absorbance of certain antibiotic steroid combinations was also studied. It has been suggested (1, 9, 13) that the polyene While studying the effects of various steroids antibiotics become bound to the fungal cell mem- on the antimonilial activity of pimaricin, we brane and cause permeability changes with observed that ergostenol was almost as effective attendant depletion of essential cellular con- as the above A5-3/3-hydroxy steroids in antag- stituents. Loss of potassium and ammonium onizing pimaricin. -

Triheptanoin for Glucose Transporter Type I Deficiency (G1D) Modulation of Human Ictogenesis, Cerebral Metabolic Rate, and Cognitive Indices by a Food Supplement
Research Original Investigation Triheptanoin for Glucose Transporter Type I Deficiency (G1D) Modulation of Human Ictogenesis, Cerebral Metabolic Rate, and Cognitive Indices by a Food Supplement Juan M. Pascual, MD, PhD; Peiying Liu, PhD; Deng Mao, BS; Dorothy I. Kelly, MA; Ana Hernandez, MS; Min Sheng, PhD; Levi B. Good, PhD; Qian Ma, MD, PhD; Isaac Marin-Valencia, MD, MS; Xuchen Zhang, MD; Jason Y. Park, MD, PhD; Linda S. Hynan, PhD; Peter Stavinoha, PhD; Charles R. Roe, MD; Hanzhang Lu, PhD Supplemental content at IMPORTANCE Disorders of brain metabolism are multiform in their mechanisms and jamaneurology.com manifestations, many of which remain insufficiently understood and are thus similarly treated. Glucose transporter type I deficiency (G1D) is commonly associated with seizures and with electrographic spike-waves. The G1D syndrome has long been attributed to energy (ie, adenosine triphosphate synthetic) failure such as that consequent to tricarboxylic acid (TCA) cycle intermediate depletion. Indeed, glucose and other substrates generate TCAs via anaplerosis. However, TCAs are preserved in murine G1D, rendering energy-failure inferences premature and suggesting a different hypothesis, also grounded on our work, that consumption of alternate TCA precursors is stimulated and may be detrimental. Second, common ketogenic diets lead to a therapeutically counterintuitive reduction in blood glucose available to the G1D brain and prove ineffective in one-third of patients. OBJECTIVE To identify the most helpful outcomes for treatment evaluation and to uphold (rather than diminish) blood glucose concentration and stimulate the TCA cycle, including anaplerosis, in G1D using the medium-chain, food-grade triglyceride triheptanoin. DESIGN, SETTING, AND PARTICIPANTS Unsponsored, open-label cases series conducted in an academic setting. -

BB 451/551 Lecture 35 Highlights
Kevin Ahern's Biochemistry (BB 451/551) at Oregon State University http://oregonstate.edu/instruct/bb451/summer13/lectures/highlightsglycer... Glycerolipid and Sphingolipid Metabolism 1. Phosphatidic acid is an immediate precursor of CDP-diacylglycerol, which is a precursor of the various glycerophospholipids . CTP combines with phosphatidic acid to yield a pyrophosphate and CDP-Diacylglycerol. Activation by CDP yields a high energy activated intermediate that can be readily converted to phosphatidyl glycerophospholipids. 2. From CDP-diacylglycerol, phosphatidyl serine can be made, as canphosphatidyl ethanolamine and phosphatidyl choline. Synthesis of phosphatidyl choline from phosphatidyl ethanolamine requires methyl groups donated by S-Adenoysyl-Methionine (SAM). Loss of the methyl groups by SAM yields S-Adenosyl-Homocysteine (I incorrectly said S-adenosyl-homoserine in the lecture). 3. Phosphatidyl ethanolamine (and phosphatidyl choline - derived from phosphatidyl ethanolamine) can both be made independently of phosphatidic acid biosynthesis. For this pathway, CDP-ethanolamine is the activated intermediate and the phosphoethanolamine of it is added to diacylglycerol to form phosphatidylethanolamine. Phosphatidyl choline can be made by the same methylation scheme in point 4. 4. Sphingolipids are synthesized beginning with palmitoyl-CoA and serine. Addition of a fatty acid to the amine group yields a ceramide. Addition of sugars to a ceramide yields either a cerebroside (single sugar added) or a ganglioside (complex sugar added). 5. Deficiencies in enzymes that degrade sphingolipids (particularly cerebrosides and gangliosides) are linked to neural disorders. One such disorder is Tay-Sachs disease. 6. Cholesterol is an important component of membranes, particularly in the brain. Cholesterol can be synthesized totally from acetyl-CoA. 7. Steroids include all compounds synthesized from cholesterol. -

Steroidal Triterpenes of Cholesterol Synthesis
Molecules 2013, 18, 4002-4017; doi:10.3390/molecules18044002 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Review Steroidal Triterpenes of Cholesterol Synthesis Jure Ačimovič and Damjana Rozman * Centre for Functional Genomics and Bio-Chips, Faculty of Medicine, Institute of Biochemistry, University of Ljubljana, Zaloška 4, Ljubljana SI-1000, Slovenia; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +386-1-543-7591; Fax: +386-1-543-7588. Received: 18 February 2013; in revised form: 19 March 2013 / Accepted: 27 March 2013 / Published: 4 April 2013 Abstract: Cholesterol synthesis is a ubiquitous and housekeeping metabolic pathway that leads to cholesterol, an essential structural component of mammalian cell membranes, required for proper membrane permeability and fluidity. The last part of the pathway involves steroidal triterpenes with cholestane ring structures. It starts by conversion of acyclic squalene into lanosterol, the first sterol intermediate of the pathway, followed by production of 20 structurally very similar steroidal triterpene molecules in over 11 complex enzyme reactions. Due to the structural similarities of sterol intermediates and the broad substrate specificity of the enzymes involved (especially sterol-Δ24-reductase; DHCR24) the exact sequence of the reactions between lanosterol and cholesterol remains undefined. This article reviews all hitherto known structures of post-squalene steroidal triterpenes of cholesterol synthesis, their biological roles and the enzymes responsible for their synthesis. Furthermore, it summarises kinetic parameters of enzymes (Vmax and Km) and sterol intermediate concentrations from various tissues. Due to the complexity of the post-squalene cholesterol synthesis pathway, future studies will require a comprehensive meta-analysis of the pathway to elucidate the exact reaction sequence in different tissues, physiological or disease conditions. -

6 Minute Walk Results
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date r i 1 /1 i 22 December 2011 (22.12.2011) » 2U1 1/159634ft Al (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 31/23 (2006.01) A61P 3/00 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, (21) International Application Number: CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, PCT/US201 1/040234 DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, JL, IN, IS, JP, KE, KG, KM, KN, KP, 13 June 201 1 (13.06.201 1) KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, (25) Filing Language: English NO, NZ, OM, PE, PG, PH, PL, PT, RO, RS, RU, SC, SD, (26) Publication Language: English SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: 61/354,472 14 June 2010 (14.06.2010) US (84) Designated States (unless otherwise indicated, for every 13/159,329 13 June 201 1 (13.06.201 1) US kind of regional protection available): ARIPO (BW, GH, GM, KE, LR, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, (71) Applicant (for all designated States except US): BAY¬ ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, LOR RESEARCH INSTITUTE [US/US]; 33 10 Live TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, Oak Street, Suite 501, Dallas, TX 75204 (US). -

Chemoprevention in Kidney Cancer by Madhur Nayan
Chemoprevention in Kidney Cancer by Madhur Nayan A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy in Clinical Epidemiology, Graduate Department of Health Policy, Management, and Evaluation, in the University of Toronto © Copyright by Madhur Nayan, 2017 THESIS ABSTRACT Thesis Title: Chemoprevention in kidney cancer Degree: Doctor of Philosophy (PhD) in Clinical Epidemiology Year of Convocation: 2017 Student: Madhur Nayan Graduate Department: Health Policy, Management and Evaluation University: University of Toronto Background: This thesis is a composition of three studies that explore the role of statins in kidney cancer. Furthermore, I evaluate the potential for different interpretations from the same data depending on the method of classifying medication use. Methods: The first study was a population-based case-control study evaluating the association of statin use with risk of incident kidney cancer. The second study was a systematic review and meta-analysis reviewing the current evidence relating statins with kidney cancer survival outcomes. The final study was a population-based cohort study evaluating the association of statin use with survival. In the observational studies, I used fractional polynomials for the primary analysis to allow for a non-linear relationship between cumulative exposure and the risk of the outcome. I also compared risk estimates obtained by different methods of classifying medication exposure. Results: The population-based case-control study included 10,377 incident cases of kidney cancer and 35,939 matched controls. Increasing cumulative use of statins was not associated with kidney cancer risk. I identified 12 studies for inclusion in the systematic review and meta- analysis and found that statin use was significantly associated with markedly improved cancer- specific and overall survival. -
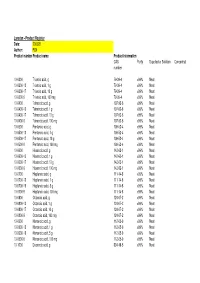
Larodan - Product Register Date: 200528 Author: FLN Product Number Product Name Product Information CAS Purity Supplied As Solution Concentration Number
Larodan - Product Register Date: 200528 Author: FLN Product number Product name Product information CAS Purity Supplied as Solution Concentration number 10-0300 Trianoic acid, g 79-09-4 >99% Neat 10-0300-13 Trianoic acid, 1 g 79-09-4 >99% Neat 10-0300-17 Trianoic acid, 10 g 79-09-4 >99% Neat 10-0300-9 Trianoic acid, 100 mg 79-09-4 >99% Neat 10-0400 Tetranoic acid, g 107-92-6 >99% Neat 10-0400-13 Tetranoic acid, 1 g 107-92-6 >99% Neat 10-0400-17 Tetranoic acid, 10 g 107-92-6 >99% Neat 10-0400-9 Tetranoic acid, 100 mg 107-92-6 >99% Neat 10-0500 Pentanoic acid, g 109-52-4 >99% Neat 10-0500-13 Pentanoic acid, 1 g 109-52-4 >99% Neat 10-0500-17 Pentanoic acid, 10 g 109-52-4 >99% Neat 10-0500-9 Pentanoic acid, 100 mg 109-52-4 >99% Neat 10-0600 Hexanoic acid, g 142-62-1 >99% Neat 10-0600-13 Hexanoic acid, 1 g 142-62-1 >99% Neat 10-0600-17 Hexanoic acid, 10 g 142-62-1 >99% Neat 10-0600-9 Hexanoic acid, 100 mg 142-62-1 >99% Neat 10-0700 Heptanoic acid, g 111-14-8 >99% Neat 10-0700-13 Heptanoic acid, 1 g 111-14-8 >99% Neat 10-0700-16 Heptanoic acid, 5 g 111-14-8 >99% Neat 10-0700-9 Heptanoic acid, 100 mg 111-14-8 >99% Neat 10-0800 Octanoic acid, g 124-07-2 >99% Neat 10-0800-13 Octanoic acid, 1 g 124-07-2 >99% Neat 10-0800-17 Octanoic acid, 10 g 124-07-2 >99% Neat 10-0800-9 Octanoic acid, 100 mg 124-07-2 >99% Neat 10-0900 Nonanoic acid, g 112-05-0 >99% Neat 10-0900-13 Nonanoic acid, 1 g 112-05-0 >99% Neat 10-0900-16 Nonanoic acid, 5 g 112-05-0 >99% Neat 10-0900-9 Nonanoic acid, 100 mg 112-05-0 >99% Neat 10-1000 Decanoic acid, g 334-48-5 >99% Neat -

Fatty Acids & Derivatives
Conditions of Sale Validity The Conditions of Sale apply to the written text in this Catalogue superseding earlier texts related to such conditions. Intention of Use Our products are intended for research purposes only. Prices See under Order Information. All prices in this catalogue are net prices in Euro, ex works. Taxes, shipping costs or other external costs demanded by the buyer are invoiced. Delivery See under Order Information – shipping terms. Payment terms Payment terms are normally net 30 days. Deductions are not accepted unless we have issued a credit note. We accept payment by credit card (Visa/ Mastercard), bank transfer (wiring) or by cheque. If payment by cheque we will add a bank fee to our invoice. Complaints Complaints about a product or products must be made inside 30 days from the invoice date. All claims must specify batch (lot) and invoice numbers. Return of goods will not be accepted unless authorized by us. Insurance Insurance will not be made unless otherwise instructed. Delays We cannot accept compensation claims due to delays or non-deliveries. We reserve us the right to withdraw from delivery due to long term shortage of starting materials, production breakdown or other circumstances beyond our control. Warranty and All products in this catalogue are warranted to be free of defects and in Compensation Claims accordance with given specifications. If this warranty does not comply with specifications, any indemnities will be limited to not exceed the price paid for the goods. Acceptance Placing of an order implies acceptance of our conditions of sale. We accept credit cards (Visa/ Mastercard) www.larodan.se [email protected] +46 40 16 41 55 1 Ordering Information You can easily order from Larodan – please contact our local distributor or us by phone, e-mail, fax or letter.