Services That Require Precertification Standard Precert Effective: 7/1/2021 This Applies to Elective, Nonemergency Services
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Genmab and ADC Therapeutics Announce Amended Agreement for Camidanlumab Tesirine (Cami)
Genmab and ADC Therapeutics Announce Amended Agreement for Camidanlumab Tesirine (Cami) Media Release Copenhagen, Denmark and Lausanne, Switzerland, October 30, 2020 • ADC Therapeutics to continue the development and commercialization of Cami • Genmab to receive mid-to-high single-digit tiered royalty Genmab A/S (Nasdaq: GMAB) and ADC Therapeutics SA (NYSE: ADCT) today announced that they have executed an amended agreement for ADC Therapeutics to continue the development and commercialization of camidanlumab tesirine (Cami). The parties first entered into a collaboration and license agreement in June 2013 for the development of Cami, an antibody drug conjugate (ADC) which combines Genmab’s HuMax®-TAC antibody targeting CD25 with ADC Therapeutics’ highly potent pyrrolobenzodiazepine (PBD) warhead technology. Under the terms of the 2013 agreement, the parties were to determine the path forward for continued development and commercialization of Cami upon completion of a Phase 1a/b clinical trial. ADC Therapeutics previously announced that Cami achieved an overall response rate of 86.5%, including a complete response rate of 48.6%, in Hodgkin lymphoma patients in this trial who had received a median of five prior lines of therapy. Cami is currently being evaluated in a 100-patient pivotal Phase 2 clinical trial intended to support the submission of a Biologics License Application (BLA) to the U.S. Food and Drug Administration (FDA). The trial is more than 50 percent enrolled and ADC Therapeutics anticipates reporting interim results in the first half of 2021. “We have a long-standing relationship with the ADC Therapeutics team and believe they are an ideal partner for the ongoing development and potential commercialization of Cami,” said Jan van de Winkel, Ph.D., Chief Executive Officer of Genmab. -

Moxetumomab Pasudotox-Tdfk
Wednesday, March 11, 2020 4:00pm Oklahoma Health Care Authority 4345 N. Lincoln Blvd. Oklahoma City, OK 73105 The University of Oklahoma Health Sciences Center COLLEGE OF PHARMACY PHARMACY MANAGEMENT CONSULTANTS MEMORANDUM TO: Drug Utilization Review (DUR) Board Members FROM: Michyla Adams, Pharm.D. SUBJECT: Packet Contents for DUR Board Meeting – March 11, 2020 DATE: February 24, 2020 NOTE: The DUR Board will meet at 4:00pm. The meeting will be held at 4345 N. Lincoln Blvd. Enclosed are the following items related to the March meeting. Material is arranged in order of the agenda. Call to Order Public Comment Forum Action Item – Approval of DUR Board Meeting Minutes – Appendix A Update on Medication Coverage Authorization Unit/SoonerPsych Program Update – Appendix B Action Item – Vote to Prior Authorize Xcopri® (Cenobamate) – Appendix C Action Item – Vote to Prior Authorize Tosymra™ (Sumatriptan Nasal Spray), Reyvow™ (Lasmiditan), and Ubrelvy™ (Ubrogepant) – Appendix D Action Item – Vote to Prior Authorize Esperoct® [Antihemophilic Factor (Recombinant), Glycopegylated-exei] – Appendix E Action Item – Vote to Prior Authorize ProAir® Digihaler™ (Albuterol Sulfate Inhalation Powder) – Appendix F Action Item – Vote to Prior Authorize Evenity® (Romosozumab-aqqg) – Appendix G Action Item – Vote to Prior Authorize Asparlas™ (Calaspargase Pegol-mknl), Daurismo™ (Glasdegib), Idhifa® (Enasidenib), Lumoxiti® (Moxetumomab Pasudotox-tdfk), Tibsovo® (Ivosidenib), and Xospata® (Gilteritinib) – Appendix H Action Item – Vote to Prior Authorize Azedra® (Iobenguane I-131) – Appendix I Annual Review of Lymphoma Medications and 30-Day Notice to Prior Authorize Aliqopa™ (Copanlisib), Brukinsa™ (Zanubrutinib), Polivy™ (Polatuzumab Vedotin-piiq), and Ruxience™ (Rituximab-pvvr) – Appendix J Annual Review of Lutathera® (Lutetium Lu-177 Dotatate) and Vitrakvi® (Larotrectinib) – Appendix K Annual Review of Multiple Sclerosis (MS) Medications and 30-Day Notice to Prior Authorize Mayzent® (Siponimod), Mavenclad® (Cladribine), and Vumerity™ (Diroximel Fumarate) – Appendix L ORI-4403 • P.O. -

Draft Minutes PDCO 12-15 November 2019
11 December 2019 EMA/PDCO/615413/2019 Inspections, Human Medicines Pharmacovigilance and Committees Division Paediatric Committee (PDCO) Minutes for the meeting on 12-15 November 2019 Chair: Koenraad Norga – Vice-Chair: Sabine Scherer Health and safety information In accordance with the Agency’s health and safety policy, delegates are to be briefed on health, safety and emergency information and procedures prior to the start of the meeting. Disclaimers Some of the information contained in these minutes is considered commercially confidential or sensitive and therefore not disclosed. With regard to intended therapeutic indications or procedure scopes listed against products, it must be noted that these may not reflect the full wording proposed by applicants and may also vary during the course of the review. Additional details on some of these procedures will be published in the PDCO Committee meeting reports (after the PDCO Opinion is adopted), and on the Opinions and decisions on paediatric investigation plans webpage (after the EMA Decision is issued). Of note, this set of minutes is a working document primarily designed for PDCO members and the work the Committee undertakes. Further information with relevant explanatory notes can be found at the end of this document. Note on access to documents Some documents mentioned in these minutes cannot be released at present following a request for access to documents within the framework of Regulation (EC) No 1049/2001 as they are subject to on- going procedures for which a final decision has not yet been adopted. They will become public when adopted or considered public according to the principles stated in the Agency policy on access to documents (EMA/127362/2006). -

Moxetumomab Pasudotox for Advanced Hairy Cell Leukemia (Enrollment Anticipated in 2018) Eligibility: • at Least 2 Prior Treatments, Including Purine Analog
Moxetumomab Pasudotox for Advanced Hairy Cell Leukemia (enrollment anticipated in 2018) Eligibility: • At least 2 prior treatments, including purine analog. • Need for treatment (low blood counts or spleen pain) • No prior recombinant toxin • Hairy cell leukemia variant (HCLv) accepted Rationale • Moxetumomab pasudotox, formally called HA22 or CAT-8015, is a recombinant immunotoxin made out of 2 parts, an antibody part binding to CD22 on B-cells, and a toxin part (domain II and III) which kills the cell. • The toxin is extremely potent, only 1 molecule in the cytoplasm is enough to kill a cell. • HCL cells have much more CD22 than normal B-cells. • Normal B-cells rapidly regenerate from CD22-negative cells, but HCL cells may not return if eradicated. • ~50% complete remission (CR) rate at the highest dose level. (https://www.ncbi.nlm.nih.gov/pubmed/22355053). • Most of these complete remissions (CRs) had no minimal residual disease (MRD) and did not relapse. • Although severe toxicity was not seen, a low-grade hemolytic uremic syndrome, with temporary decrease in platelets and increase in creatinine, was seen in 2 of 49 patients. Design • 30 minute iv infusion every other day for 3 doses, repeat every 4 weeks for 6 cycles. • Patients are then followed without treatment. Cladribine With Simultaneous or Delayed Rituximab for early HCL Cladribine (daily x5) Rituximab weekly x8 (CDAR) |||||||| |||||||| Cladribine + immediate Rituximab vs > 6 mo |||||||| Cladribine + delayed Rituximab |||||||| • Eligibility: 0-1 prior purine analog, or HCL variant (HCLv), and need for treatment (i.e. low blood counts) • Minimal residual disease (MRD) after purine analog (cladribine or pentostatin) may cause relapse. -

(CHMP) Agenda for the Meeting on 22-25 February 2021 Chair: Harald Enzmann – Vice-Chair: Bruno Sepodes
22 February 2021 EMA/CHMP/107904/2021 Human Medicines Division Committee for medicinal products for human use (CHMP) Agenda for the meeting on 22-25 February 2021 Chair: Harald Enzmann – Vice-Chair: Bruno Sepodes 22 February 2021, 09:00 – 19:30, room 1C 23 February 2021, 08:30 – 19:30, room 1C 24 February 2021, 08:30 – 19:30, room 1C 25 February 2021, 08:30 – 19:30, room 1C Disclaimers Some of the information contained in this agenda is considered commercially confidential or sensitive and therefore not disclosed. With regard to intended therapeutic indications or procedure scopes listed against products, it must be noted that these may not reflect the full wording proposed by applicants and may also vary during the course of the review. Additional details on some of these procedures will be published in the CHMP meeting highlights once the procedures are finalised and start of referrals will also be available. Of note, this agenda is a working document primarily designed for CHMP members and the work the Committee undertakes. Note on access to documents Some documents mentioned in the agenda cannot be released at present following a request for access to documents within the framework of Regulation (EC) No 1049/2001 as they are subject to on- going procedures for which a final decision has not yet been adopted. They will become public when adopted or considered public according to the principles stated in the Agency policy on access to documents (EMA/127362/2006). Official address Domenico Scarlattilaan 6 ● 1083 HS Amsterdam ● The Netherlands Address for visits and deliveries Refer to www.ema.europa.eu/how-to-find-us Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 781 6000 An agency of the European Union © European Medicines Agency, 2021. -

Keeping up with FDA Drug Approvals: 60 New Drugs in 60 Minutes Elizabeth A
Keeping Up with FDA Drug Approvals: 60 New Drugs in 60 Minutes Elizabeth A. Shlom, PharmD, BCPS Senior Vice President & Director Clinical Pharmacy Program | Acurity, Inc. Privileged and Confidential April 10, 2019 Privileged and Confidential Program Objectives By the end of the presentation, the pharmacist or pharmacy technician participant will be able to: ▪ Identify orphan drugs and first-in-class medications approved by the FDA in 2018. ▪ List five new drugs and their indications. ▪ Identify the place in therapy for three novel monoclonal antibodies. ▪ Discuss at least two new medications that address public health concerns. Dr. Shlom does not have any conflicts of interest in regard to this presentation. Both trade names and generic names will be discussed throughout the presentation Privileged and Confidential 2018 NDA Approvals (NMEs/BLAs) ▪ Lutathera (lutetium Lu 177 dotatate) ▪ Braftovi (encorafenib) ▪ Vizimpro (dacomitinib) ▪ Biktarvy (bictegravir, emtricitabine, ▪ TPOXX (tecovirimat) ▪ Libtayo (cemiplimab-rwic) tenofovir, ▪ Tibsovo (ivosidenib) ▪ Seysara (sarecycline) alafenamide) ▪ Krintafel (tafenoquine) ▪ Nuzyra (omadacycline) ▪ Symdeko (tezacaftor, ivacaftor) ▪ Orilissa (elagolix sodium) ▪ Revcovi (elapegademase-lvir) ▪ Erleada (apalutamide) ▪ Omegaven (fish oil triglycerides) ▪ Tegsedi (inotersen) ▪ Trogarzo (ibalizumab-uiyk) ▪ Mulpleta (lusutrombopag) ▪ Talzenna (talazoparib) ▪ Ilumya (tildrakizumab-asmn) ▪ Poteligeo (mogamulizumab-kpkc) ▪ Xofluza (baloxavir marboxil) ▪ Tavalisse (fostamatinib disodium) ▪ Onpattro (patisiran) -

Antibody–Drug Conjugates
Published OnlineFirst April 12, 2019; DOI: 10.1158/1078-0432.CCR-19-0272 Review Clinical Cancer Research Antibody–Drug Conjugates: Future Directions in Clinical and Translational Strategies to Improve the Therapeutic Index Steven Coats1, Marna Williams1, Benjamin Kebble1, Rakesh Dixit1, Leo Tseng1, Nai-Shun Yao1, David A. Tice1, and Jean-Charles Soria1,2 Abstract Since the first approval of gemtuzumab ozogamicin nism of activity of the cytotoxic warhead. However, the (Mylotarg; Pfizer; CD33 targeted), two additional antibody– enthusiasm to develop ADCs has not been dampened; drug conjugates (ADC), brentuximab vedotin (Adcetris; Seat- approximately 80 ADCs are in clinical development in tle Genetics, Inc.; CD30 targeted) and inotuzumab ozogami- nearly 600 clinical trials, and 2 to 3 novel ADCs are likely cin (Besponsa; Pfizer; CD22 targeted), have been approved for to be approved within the next few years. While the hematologic cancers and 1 ADC, trastuzumab emtansine promise of a more targeted chemotherapy with less tox- (Kadcyla; Genentech; HER2 targeted), has been approved to icity has not yet been realized with ADCs, improvements treat breast cancer. Despite a clear clinical benefit being dem- in technology combined with a wealth of clinical data are onstrated for all 4 approved ADCs, the toxicity profiles are helping to shape the future development of ADCs. In this comparable with those of standard-of-care chemotherapeu- review, we discuss the clinical and translational strategies tics, with dose-limiting toxicities associated with the mecha- associated with improving the therapeutic index for ADCs. Introduction in antibody, linker, and warhead technologies in significant depth (2, 3, 8, 9). Antibody–drug conjugates (ADC) were initially designed to leverage the exquisite specificity of antibodies to deliver targeted potent chemotherapeutic agents with the intention of improving Overview of ADCs in Clinical Development the therapeutic index (the ratio between the toxic dose and the Four ADCs have been approved over the last 20 years (Fig. -

SGO-2020-Annual-Meet
Society of Gynecologic Oncology 2020 Annual Meeting on Women’s Cancer Abstracts for Oral Presentation Scientific Plenary I: Shaping the Future with Innovative Clinical Trials: A Clearer Vision Ahead in Gynecologic Cancer 1 - Scientific Plenary Sentinel lymph node biopsy versus lymphadenectomy for high-grade endometrial cancer staging (SENTOR trial): A prospective multicenter cohort study M.C. Cusimanoa, D. Vicusb, K. Pulmanc, M.Q. Bernardinid, S. Laframboised, T. Mayd, G. Bouchard-Fortierd, L. Hogend, L.T. Gienb, A.L. Covensb, R. Kupetse, B.A. Clarkea, M. Cesaric, M. Rouzbahmand, J. Mirkovicb, G. Turashvilid, M. Magantid, A. Ziad, G.E.V. Ened and S.E. Fergusond. aUniversity of Toronto, Toronto, ON, Canada, bSunnybrook Odette Cancer Centre, Toronto, ON, Canada, cTrillium Health Partners, Credit Valley Hospital/University of Toronto, Mississauga, ON, Canada, dPrincess Margaret Cancer Centre, University Health Network, Toronto, ON, Canada, eSunnybrook Cancer Centre/University of Toronto, Toronto, ON, Canada Objective: It is unclear whether sentinel lymph node biopsy (SLNB) can replace complete lymphadenectomy in women with high-grade endometrial cancer (EC). We performed a prospective multicenter cohort study (the SENTOR trial) to evaluate the performance characteristics of SLNB using indocyanine green (ICG) in stage I high-grade EC (ClinicalTrials.gov ID: NCT01886066). Method: Patients with clinical stage I grade 2 endometrioid or high-grade EC (grade 3 endometrioid, serous, clear cell, carcinosarcoma, undifferentiated, or mixed tumors) undergoing laparoscopic or robotic surgery at 3 cancer centers in Toronto, Canada, were prospectively recruited for SLNB with ICG. After SLNB, high-grade EC patients underwent pelvic and paraaortic lymphadenectomy (PLND/PALND), and grade 2 endometrioid EC patients underwent PLND only. -

Targeting the Epidermal Growth Factor Receptor in EGFR-Mutated Lung Cancer: Current and Emerging Therapies
cancers Review Targeting the Epidermal Growth Factor Receptor in EGFR-Mutated Lung Cancer: Current and Emerging Therapies Karam Khaddour 1,*, Sushma Jonna 1, Alexander Deneka 2 , Jyoti D. Patel 3, Mohamed E. Abazeed 4, Erica Golemis 2 , Hossein Borghaei 5 and Yanis Boumber 3,6,* 1 Division of Hematology and Oncology, University of Illinois at Chicago, Chicago, IL 60612, USA; [email protected] 2 Fox Chase Cancer Center, Program in Molecular Therapeutics, Philadelphia, PA 19111, USA; [email protected] (A.D.); [email protected] (E.G.) 3 Robert H. Lurie Comprehensive Cancer Center, Division of Hematology/Oncology, Feinberg School of Medicine, Northwestern University, Chicago, IL 60611, USA; [email protected] 4 Robert H. Lurie Comprehensive Cancer Center, Department of Radiation Oncology, Feinberg School of Medicine, Northwestern University, Chicago, IL 60611, USA; [email protected] 5 Fox Chase Cancer Center, Department of Hematology and Oncology, Philadelphia, PA 19111, USA; [email protected] 6 Institute of Fundamental Medicine and Biology, Kazan Federal University, 420008 Kazan, Russia * Correspondence: [email protected] (K.K.); [email protected] (Y.B.) Simple Summary: Epidermal growth factor receptor (EGFR) mutations occur in a significant number Citation: Khaddour, K.; Jonna, S.; of lung cancer patients. Treatment outcomes in this subset of patients has greatly improved over the Deneka, A.; Patel, J.D.; Abazeed, M.E.; last decade after the introduction of EGFR tyrosine kinase inhibitors (TKIs), which demonstrated high Golemis, E.; Borghaei, H.; Boumber, Y. efficacy and improved survival in randomized clinical trials. Although EGFR TKIs became the stan- Targeting the Epidermal Growth dard of care in patients with EGFR-mutated lung cancer, resistance almost inevitably develops. -

Interim Report for the First Quarter of 2020
Genmab Announces Financial Results for the First Quarter of 2020 May 6, 2020; Copenhagen, Denmark; Interim Report for the First Quarter Ended March 31, 2020 Highlights DARZALEX® (daratumumab) net sales increased approximately 49% compared to the first quarter of 2019 to USD 937 million, resulting in royalty income of DKK 775 million DARZALEX approved in Europe in combination with bortezomib, thalidomide and dexamethasone for the treatment of adult patients with newly diagnosed multiple myeloma who are eligible for autologous stem cell transplant U.S. FDA approved TEPEZZA™ (teprotumumab-trbw), developed and commercialized by Horizon Therapeutics, for thyroid eye disease U.S. FDA accepted, with priority review, Novartis’ supplemental Biologics License Application for subcutaneous ofatumumab in relapsing multiple sclerosis Anthony Pagano appointed Chief Financial Officer Anthony Mancini appointed Chief Operating Officer “Despite the unprecedented challenges posed by the coronavirus (COVID-19) pandemic, we will continue to invest in our innovative proprietary products, technologies and capabilities and use our world-class expertise in antibody drug development to create truly differentiated products with the potential to help cancer patients. While Genmab is closely monitoring the developments in the rapidly evolving landscape, we are extremely fortunate to have a solid financial foundation and a fabulous and committed team to carry us through these uncertain times,” said Jan van de Winkel, Ph.D., Chief Executive Officer of Genmab. Financial Performance First Quarter of 2020 Revenue was DKK 892 million in the first quarter of 2020 compared to DKK 591 million in the first quarter of 2019. The increase of DKK 301 million, or 51%, was mainly driven by higher DARZALEX royalties. -

Eflapegrastim-Xnst Submitted by Spectrum Pharmaceuticals
Anton F. Ehrhardt, PhD, VP Medical Affairs Spectrum Pharmaceuticals, Inc One Main St, 11th floor Cambridge, MA 02142 Phone: (617) 477-8091 Email: [email protected] Date of request: 31 August 2020 NCCN Guidelines Panel: Hematopoietic Growth Factors On behalf of Spectrum Pharmaceuticals, Inc., I respectfully request the NCCN Hematopoietic Growth Factors Panel review the enclosed data for inclusion of eflapegrastim-xnst (Rolontis®), a non-biosimilar long-acting G- CSF of noVel structure, as a recommendation for prophylaxis of febrile neutropenia and maintenance of scheduled dose deliVery (MGF-B). Specific Changes: 1. Addition of eflapegrastim-xnst as a recommendation for prophylaxis of febrile neutropenia and maintenance of scheduled dose deliVery (MGF-B) a. Dosing recommendation below listing of eflapegrastim-xnst: One dose of 13.2 mg (MGF-B) 2. Addition of eflapegrastim-xnst to the listing of Filgrastim, Pegfilgrastim and Tbo-filgrastim in table MGF- D (Toxicity Risks for Myeloid Growth Factors) FDA Clearance: Rolontis is undergoing FDA reView based on an original BLA#761148 for a proposed indication of: to decrease the incidence of infection, as manifested by febrile neutropenia, in patients with non-myeloid malignancies receiving myelosuppressive anti-cancer drugs associated with clinically significant incidence of febrile neutropenia. PDUFA for this reView is October 24th, 2020. Rationale: In support of the proposed change, data were generated in pre-clinical in-Vitro and animal model studies that indicated structure-related enhancement of potency, and increased concentrations in bone marrow compared to pegfilgrastim (Barrett 2020(reference 1)). Eflapegrastim is composed of a recombinant human G- CSF joined to an IgG4 Fc moiety Via a short polyethylene glycol linker. -
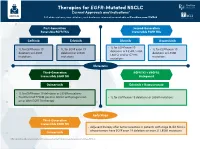
Therapies for EGFR-Mutated NSCLC Current Approvals and Indications1 Full Abbreviations, Accreditation, and Disclosure Information Available at Peerview.Com/CWE40
Therapies for EGFR-Mutated NSCLC 1 Current Approvals and Indications Full abbreviations, accreditation, and disclosure information available at PeerView.com/CWE40 First-Generation Second-Generation Reversible EGFR TKIs Irreversible EGFR TKIs Getinib Erlotinib Afatinib Dacomitinib • 1L for EGFR exon 19 • 1L for EGFR exon 19 • 1L for EGFR exon 19 • 1L for EGFR exon 19 deletions or L858R, S768I, deletions or L858R deletions or L858R deletions or L858R L861Q, and/or G719X mutations mutations mutations mutations Metastatic Third-Generation EGFR TKI + VEGFR2 Irreversible EGFR TKI Antagonist Osimertinib Erlotinib + Ramucirumab • 1L for EGFR exon 19 deletions or L858R mutations • Treatment of T790M-positive NSCLC with progression • 1L for EGFR exon 19 deletions or L858R mutations on or after EGFR TKI therapy Early Stage Third-Generation Irreversible EGFR TKI • Adjuvant therapy after tumor resection in patients with stage IB-IIIA NSCLC whose tumors have EGFR exon 19 deletions or exon 21 L858R mutations Osimertinib 1. https://www.fda.gov/drugs/resources-information-approved-drugs/hematologyoncology-cancer-approvals-safety-notifications. Molecular Testing Guidelines for NSCLC Latest Updates, Best Practices, and Patient-Reported Insights1 Full abbreviations, accreditation, and disclosure information available at PeerView.com/CWE40 Why Test Lung Cancer Patients for Genomic Alterations? • Genomic alterations are common in nonsquamous NSCLC (approximately 50%) • Targeted therapies produce better treatment outcomes (eg, higher response rates, improved