Quantum Dynamics Simulation of Electrons in Materials on High-Performance Computers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Free and Open Source Software for Computational Chemistry Education
Free and Open Source Software for Computational Chemistry Education Susi Lehtola∗,y and Antti J. Karttunenz yMolecular Sciences Software Institute, Blacksburg, Virginia 24061, United States zDepartment of Chemistry and Materials Science, Aalto University, Espoo, Finland E-mail: [email protected].fi Abstract Long in the making, computational chemistry for the masses [J. Chem. Educ. 1996, 73, 104] is finally here. We point out the existence of a variety of free and open source software (FOSS) packages for computational chemistry that offer a wide range of functionality all the way from approximate semiempirical calculations with tight- binding density functional theory to sophisticated ab initio wave function methods such as coupled-cluster theory, both for molecular and for solid-state systems. By their very definition, FOSS packages allow usage for whatever purpose by anyone, meaning they can also be used in industrial applications without limitation. Also, FOSS software has no limitations to redistribution in source or binary form, allowing their easy distribution and installation by third parties. Many FOSS scientific software packages are available as part of popular Linux distributions, and other package managers such as pip and conda. Combined with the remarkable increase in the power of personal devices—which rival that of the fastest supercomputers in the world of the 1990s—a decentralized model for teaching computational chemistry is now possible, enabling students to perform reasonable modeling on their own computing devices, in the bring your own device 1 (BYOD) scheme. In addition to the programs’ use for various applications, open access to the programs’ source code also enables comprehensive teaching strategies, as actual algorithms’ implementations can be used in teaching. -

Basis Sets Running a Calculation • in Performing a Computational
Session 2: Basis Sets • Two of the major methods (ab initio and DFT) require some understanding of basis sets and basis functions • This session describes the essentials of basis sets: – What they are – How they are constructed – How they are used – Significance in choice 1 Running a Calculation • In performing a computational chemistry calculation, the chemist has to make several decisions of input to the code: – The molecular geometry (and spin state) – The basis set used to determine the wavefunction – The properties to be calculated – The type(s) of calculations and any accompanying assumptions 2 Running a calculation •For ab initio or DFT calculations, many programs require a basis set choice to be made – The basis set is an approx- imate representation of the atomic orbitals (AOs) – The program then calculates molecular orbitals (MOs) using the Linear Combin- ation of Atomic Orbitals (LCAO) approximation 3 Computational Chemistry Map Chemist Decides: Computer calculates: Starting Molecular AOs determine the Geometry AOs O wavefunction (ψ) Basis Set (with H H ab initio and DFT) Type of Calculation LCAO (Method and Assumptions) O Properties to HH be Calculated MOs 4 Critical Choices • Choice of the method (and basis set) used is critical • Which method? • Molecular Mechanics, Ab initio, Semiempirical, or DFT • Which approximation? • MM2, MM3, HF, AM1, PM3, or B3LYP, etc. • Which basis set (if applicable)? • Minimal basis set • Split-valence • Polarized, Diffuse, High Angular Momentum, ...... 5 Why is Basis Set Choice Critical? • The -

Quan Wan, "First Principles Simulations of Vibrational Spectra
THE UNIVERSITY OF CHICAGO FIRST PRINCIPLES SIMULATIONS OF VIBRATIONAL SPECTRA OF AQUEOUS SYSTEMS A DISSERTATION SUBMITTED TO THE FACULTY OF THE INSTITUTE FOR MOLECULAR ENGINEERING IN CANDIDACY FOR THE DEGREE OF DOCTOR OF PHILOSOPHY BY QUAN WAN CHICAGO, ILLINOIS AUGUST 2015 Copyright c 2015 by Quan Wan All Rights Reserved For Jing TABLE OF CONTENTS LISTOFFIGURES .................................... vii LISTOFTABLES ..................................... xii ACKNOWLEDGMENTS ................................. xiii ABSTRACT ........................................ xiv 1 INTRODUCTION ................................... 1 2 FIRST PRINCIPLES CALCULATIONS OF GROUND STATE PROPERTIES . 5 2.1 Densityfunctionaltheory. 5 2.1.1 TheSchr¨odingerEquation . 5 2.1.2 Density Functional Theory and the Kohn-Sham Ansatz . .. 6 2.1.3 Approximations to the Exchange-Correlation Energy Functional... 9 2.1.4 Planewave Pseudopotential Scheme . 11 2.1.5 First Principles Molecular Dynamics . 12 3 FIRST-PRINCIPLES CALCULATIONS IN THE PRESENCE OF EXTERNAL ELEC- TRICFIELDS ..................................... 14 3.1 Density functional perturbation theory for Homogeneous Electric Field Per- turbations..................................... 14 3.1.1 LinearResponsewithinDFPT. 14 3.1.2 Homogeneous Electric Field Perturbations . 15 3.1.3 ImplementationofDFPTintheQboxCode . 17 3.2 Finite Field Methods for Homogeneous Electric Field . 20 3.2.1 Finite Field Methods . 21 3.2.2 Computational Details of Finite Field Implementations . 25 3.2.3 Results for Single Water Molecules and Liquid Water . 27 3.2.4 Conclusions ................................ 32 4 VIBRATIONAL SIGNATURES OF CHARGE FLUCTUATIONS IN THE HYDRO- GENBONDNETWORKOFLIQUIDWATER . 34 4.1 IntroductiontoVibrationalSpectroscopy . ..... 34 4.1.1 NormalModeandTCFApproaches. 35 4.1.2 InfraredSpectroscopy. .. .. 36 4.1.3 RamanSpectroscopy ........................... 37 4.2 RamanSpectroscopyforLiquidWater . 39 4.3 TheoreticalMethods ............................... 41 4.3.1 Simulation Details . 41 4.3.2 Effective molecular polarizabilities . -

An Introduction to Hartree-Fock Molecular Orbital Theory
An Introduction to Hartree-Fock Molecular Orbital Theory C. David Sherrill School of Chemistry and Biochemistry Georgia Institute of Technology June 2000 1 Introduction Hartree-Fock theory is fundamental to much of electronic structure theory. It is the basis of molecular orbital (MO) theory, which posits that each electron's motion can be described by a single-particle function (orbital) which does not depend explicitly on the instantaneous motions of the other electrons. Many of you have probably learned about (and maybe even solved prob- lems with) HucÄ kel MO theory, which takes Hartree-Fock MO theory as an implicit foundation and throws away most of the terms to make it tractable for simple calculations. The ubiquity of orbital concepts in chemistry is a testimony to the predictive power and intuitive appeal of Hartree-Fock MO theory. However, it is important to remember that these orbitals are mathematical constructs which only approximate reality. Only for the hydrogen atom (or other one-electron systems, like He+) are orbitals exact eigenfunctions of the full electronic Hamiltonian. As long as we are content to consider molecules near their equilibrium geometry, Hartree-Fock theory often provides a good starting point for more elaborate theoretical methods which are better approximations to the elec- tronic SchrÄodinger equation (e.g., many-body perturbation theory, single-reference con¯guration interaction). So...how do we calculate molecular orbitals using Hartree-Fock theory? That is the subject of these notes; we will explain Hartree-Fock theory at an introductory level. 2 What Problem Are We Solving? It is always important to remember the context of a theory. -

User Manual for the Uppsala Quantum Chemistry Package UQUANTCHEM V.35
User manual for the Uppsala Quantum Chemistry package UQUANTCHEM V.35 by Petros Souvatzis Uppsala 2016 Contents 1 Introduction 6 2 Compiling the code 7 3 What Can be done with UQUANTCHEM 9 3.1 Hartree Fock Calculations . 9 3.2 Configurational Interaction Calculations . 9 3.3 M¨ollerPlesset Calculations (MP2) . 9 3.4 Density Functional Theory Calculations (DFT)) . 9 3.5 Time Dependent Density Functional Theory Calculations (TDDFT)) . 10 3.6 Quantum Montecarlo Calculations . 10 3.7 Born Oppenheimer Molecular Dynamics . 10 4 Setting up a UQANTCHEM calculation 12 4.1 The input files . 12 4.1.1 The INPUTFILE-file . 12 4.1.2 The BASISFILE-file . 13 4.1.3 The BASISFILEAUX-file . 14 4.1.4 The DENSMATSTARTGUESS.dat-file . 14 4.1.5 The MOLDYNRESTART.dat-file . 14 4.1.6 The INITVELO.dat-file . 15 4.1.7 Running Uquantchem . 15 4.2 Input parameters . 15 4.2.1 CORRLEVEL ................................. 15 4.2.2 ADEF ..................................... 15 4.2.3 DOTDFT ................................... 16 4.2.4 NSCCORR ................................... 16 4.2.5 SCERR .................................... 16 4.2.6 MIXTDDFT .................................. 16 4.2.7 EPROFILE .................................. 16 4.2.8 DOABSSPECTRUM ............................... 17 4.2.9 NEPERIOD .................................. 17 4.2.10 EFIELDMAX ................................. 17 4.2.11 EDIR ..................................... 17 4.2.12 FIELDDIR .................................. 18 4.2.13 OMEGA .................................... 18 2 CONTENTS 3 4.2.14 NCHEBGAUSS ................................. 18 4.2.15 RIAPPROX .................................. 18 4.2.16 LIMPRECALC (Only openmp-version) . 19 4.2.17 DIAGDG ................................... 19 4.2.18 NLEBEDEV .................................. 19 4.2.19 MOLDYN ................................... 19 4.2.20 DAMPING ................................... 19 4.2.21 XLBOMD .................................. -

Density Functional Theory
Density Functional Theory Fundamentals Video V.i Density Functional Theory: New Developments Donald G. Truhlar Department of Chemistry, University of Minnesota Support: AFOSR, NSF, EMSL Why is electronic structure theory important? Most of the information we want to know about chemistry is in the electron density and electronic energy. dipole moment, Born-Oppenheimer charge distribution, approximation 1927 ... potential energy surface molecular geometry barrier heights bond energies spectra How do we calculate the electronic structure? Example: electronic structure of benzene (42 electrons) Erwin Schrödinger 1925 — wave function theory All the information is contained in the wave function, an antisymmetric function of 126 coordinates and 42 electronic spin components. Theoretical Musings! ● Ψ is complicated. ● Difficult to interpret. ● Can we simplify things? 1/2 ● Ψ has strange units: (prob. density) , ● Can we not use a physical observable? ● What particular physical observable is useful? ● Physical observable that allows us to construct the Hamiltonian a priori. How do we calculate the electronic structure? Example: electronic structure of benzene (42 electrons) Erwin Schrödinger 1925 — wave function theory All the information is contained in the wave function, an antisymmetric function of 126 coordinates and 42 electronic spin components. Pierre Hohenberg and Walter Kohn 1964 — density functional theory All the information is contained in the density, a simple function of 3 coordinates. Electronic structure (continued) Erwin Schrödinger -
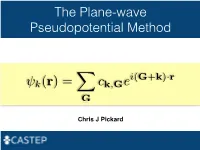
The Plane-Wave Pseudopotential Method
The Plane-wave Pseudopotential Method i(G+k) r k(r)= ck,Ge · XG Chris J Pickard Electrons in a Solid Nearly Free Electrons Nearly Free Electrons Nearly Free Electrons Electronic Structures Methods Empirical Pseudopotentials Ab Initio Pseudopotentials Ab Initio Pseudopotentials Ab Initio Pseudopotentials Ab Initio Pseudopotentials Ultrasoft Pseudopotentials (Vanderbilt, 1990) Projector Augmented Waves Deriving the Pseudo Hamiltonian PAW vs PAW C. G. van de Walle and P. E. Bloechl 1993 (I) PAW to calculate all electron properties from PSP calculations" P. E. Bloechl 1994 (II)! The PAW electronic structure method" " The two are linked, but should not be confused References David Vanderbilt, Soft self-consistent pseudopotentials in a generalised eigenvalue formalism, PRB 1990 41 7892" Kari Laasonen et al, Car-Parinello molecular dynamics with Vanderbilt ultrasoft pseudopotentials, PRB 1993 47 10142" P.E. Bloechl, Projector augmented-wave method, PRB 1994 50 17953" G. Kresse and D. Joubert, From ultrasoft pseudopotentials to the projector augmented-wave method, PRB 1999 59 1758 On-the-fly Pseudopotentials - All quantities for PAW (I) reconstruction available" - Allows automatic consistency of the potentials with functionals" - Even fewer input files required" - The code in CASTEP is a cut down (fast) pseudopotential code" - There are internal databases" - Other generation codes (Vanderbilt’s original, OPIUM, LD1) exist It all works! Kohn-Sham Equations Being (Self) Consistent Eigenproblem in a Basis Just a Few Atoms In a Crystal Plane-waves -

Introduction to DFT and the Plane-Wave Pseudopotential Method
Introduction to DFT and the plane-wave pseudopotential method Keith Refson STFC Rutherford Appleton Laboratory Chilton, Didcot, OXON OX11 0QX 23 Apr 2014 Parallel Materials Modelling Packages @ EPCC 1 / 55 Introduction Synopsis Motivation Some ab initio codes Quantum-mechanical approaches Density Functional Theory Electronic Structure of Condensed Phases Total-energy calculations Introduction Basis sets Plane-waves and Pseudopotentials How to solve the equations Parallel Materials Modelling Packages @ EPCC 2 / 55 Synopsis Introduction A guided tour inside the “black box” of ab-initio simulation. Synopsis • Motivation • The rise of quantum-mechanical simulations. Some ab initio codes Wavefunction-based theory • Density-functional theory (DFT) Quantum-mechanical • approaches Quantum theory in periodic boundaries • Plane-wave and other basis sets Density Functional • Theory SCF solvers • Molecular Dynamics Electronic Structure of Condensed Phases Recommended Reading and Further Study Total-energy calculations • Basis sets Jorge Kohanoff Electronic Structure Calculations for Solids and Molecules, Plane-waves and Theory and Computational Methods, Cambridge, ISBN-13: 9780521815918 Pseudopotentials • Dominik Marx, J¨urg Hutter Ab Initio Molecular Dynamics: Basic Theory and How to solve the Advanced Methods Cambridge University Press, ISBN: 0521898633 equations • Richard M. Martin Electronic Structure: Basic Theory and Practical Methods: Basic Theory and Practical Density Functional Approaches Vol 1 Cambridge University Press, ISBN: 0521782856 -

“Band Structure” for Electronic Structure Description
www.fhi-berlin.mpg.de Fundamentals of heterogeneous catalysis The very basics of “band structure” for electronic structure description R. Schlögl www.fhi-berlin.mpg.de 1 Disclaimer www.fhi-berlin.mpg.de • This lecture is a qualitative introduction into the concept of electronic structure descriptions different from “chemical bonds”. • No mathematics, unfortunately not rigorous. • For real understanding read texts on solid state physics. • Hellwege, Marfunin, Ibach+Lüth • The intention is to give an impression about concepts of electronic structure theory of solids. 2 Electronic structure www.fhi-berlin.mpg.de • For chemists well described by Lewis formalism. • “chemical bonds”. • Theorists derive chemical bonding from interaction of atoms with all their electrons. • Quantum chemistry with fundamentally different approaches: – DFT (density functional theory), see introduction in this series – Wavefunction-based (many variants). • All solve the Schrödinger equation. • Arrive at description of energetics and geometry of atomic arrangements; “molecules” or unit cells. 3 Catalysis and energy structure www.fhi-berlin.mpg.de • The surface of a solid is traditionally not described by electronic structure theory: • No periodicity with the bulk: cluster or slab models. • Bulk structure infinite periodically and defect-free. • Surface electronic structure theory independent field of research with similar basics. • Never assume to explain reactivity with bulk electronic structure: accuracy, model assumptions, termination issue. • But: electronic -

Simple Molecular Orbital Theory Chapter 5
Simple Molecular Orbital Theory Chapter 5 Wednesday, October 7, 2015 Using Symmetry: Molecular Orbitals One approach to understanding the electronic structure of molecules is called Molecular Orbital Theory. • MO theory assumes that the valence electrons of the atoms within a molecule become the valence electrons of the entire molecule. • Molecular orbitals are constructed by taking linear combinations of the valence orbitals of atoms within the molecule. For example, consider H2: 1s + 1s + • Symmetry will allow us to treat more complex molecules by helping us to determine which AOs combine to make MOs LCAO MO Theory MO Math for Diatomic Molecules 1 2 A ------ B Each MO may be written as an LCAO: cc11 2 2 Since the probability density is given by the square of the wavefunction: probability of finding the ditto atom B overlap term, important electron close to atom A between the atoms MO Math for Diatomic Molecules 1 1 S The individual AOs are normalized: 100% probability of finding electron somewhere for each free atom MO Math for Homonuclear Diatomic Molecules For two identical AOs on identical atoms, the electrons are equally shared, so: 22 cc11 2 2 cc12 In other words: cc12 So we have two MOs from the two AOs: c ,1() 1 2 c ,1() 1 2 2 2 After normalization (setting d 1 and _ d 1 ): 1 1 () () [2(1 S )]1/2 12 [2(1 S )]1/2 12 where S is the overlap integral: 01 S LCAO MO Energy Diagram for H2 H2 molecule: two 1s atomic orbitals combine to make one bonding and one antibonding molecular orbital. -

Qbox (Qb@Ll Branch)
Qbox (qb@ll branch) Summary Version 1.2 Purpose of Benchmark Qbox is a first-principles molecular dynamics code used to compute the properties of materials directly from the underlying physics equations. Density Functional Theory is used to provide a quantum-mechanical description of chemically-active electrons and nonlocal norm-conserving pseudopotentials are used to represent ions and core electrons. The method has O(N3) computational complexity, where N is the total number of valence electrons in the system. Characteristics of Benchmark The computational workload of a Qbox run is split between parallel dense linear algebra, carried out by the ScaLAPACK library, and a custom 3D Fast Fourier Transform. The primary data structure, the electronic wavefunction, is distributed across a 2D MPI process grid. The shape of the process grid is defined by the nrowmax variable set in the Qbox input file, which sets the number of process rows. Parallel performance can vary significantly with process grid shape, although past experience has shown that highly rectangular process grids (e.g. 2048 x 64) give the best performance. The majority of the run time is spent in matrix multiplication, Gram-Schmidt orthogonalization and parallel FFTs. Several ScaLAPACK routines do not scale well beyond 100k+ MPI tasks, so threading can be used to run on more cores. The parallel FFT and a few other loops are threaded in Qbox with OpenMP, but most of the threading speedup comes from the use of threaded linear algebra kernels (blas, lapack, ESSL). Qbox requires a minimum of 1GB of main memory per MPI task. Mechanics of Building Benchmark To compile Qbox: 1. -

Powerpoint Presentation
Session 2 Basis Sets CCCE 2008 1 Session 2: Basis Sets • Two of the major methods (ab initio and DFT) require some understanding of basis sets and basis functions • This session describes the essentials of basis sets: – What they are – How they are constructed – How they are used – Significance in choice CCCE 2008 2 Running a Calculation • In performing ab initio and DFT computa- tional chemistry calculations, the chemist has to make several decisions of input to the code: – The molecular geometry (and spin state) – The basis set used to determine the wavefunction – The properties to be calculated – The type(s) of calculations and any accompanying assumptions CCCE 2008 3 Running a calculation • For ab initio or DFT calculations, many programs require a basis set choice to be made – The basis set is an approx- imate representation of the atomic orbitals (AOs) – The program then calculates molecular orbitals (MOs) using the Linear Combin- ation of Atomic Orbitals (LCAO) approximation CCCE 2008 4 Computational Chemistry Map Chemist Decides: Computer calculates: Starting Molecular AOs determine the Geometry AOs O wavefunction (ψ) Basis Set (with H H ab initio and DFT) Type of Calculation LCAO (Method and Assumptions) O Properties to HH be Calculated MOs CCCE 2008 5 Critical Choices • Choice of the method (and basis set) used is critical • Which method? • Molecular Mechanics, Ab initio, Semiempirical, or DFT • Which approximation? • MM2, MM3, HF, AM1, PM3, or B3LYP, etc. • Which basis set (if applicable)? • Minimal basis set • Split-valence