Molds Aboard the International Space Station
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Soyuz TMA-11 / Expedition 16 Manuel De La Mission
Soyuz TMA-11 / Expedition 16 Manuel de la mission SOYUZ TMA-11 – EXPEDITION 16 Par Philippe VOLVERT SOMMAIRE I. Présentation des équipages II. Présentation de la mission III. Présentation du vaisseau Soyuz IV. Précédents équipages de l’ISS V. Chronologie de lancement VI. Procédures d’amarrage VII. Procédures de retour VIII. Horaires IX. Sources A noter que toutes les heures présentes dans ce dossier sont en heure GMT. I. PRESENTATION DES EQUIPAGES Equipage Expedition 15 Fyodor YURCHIKHIN (commandant ISS) Lieu et Lieu et date de naissance : 03/01/1959 ; Batumi (Géorgie) Statut familial : Marié et 2 enfants Etudes : Graduat d’économie à la Moscow Service State University Statut professionnel: Ingénieur et travaille depuis 1993 chez RKKE Roskosmos : Sélectionné le 28/07/1997 (RKKE-13) Précédents vols : STS-112 (07/10/2002 au 18/10/2002), totalisant 10 jours 19h58 Oleg KOTOV(ingénieur de bord) Lieu et date de naissance : 27/10/1965 ; Simferopol (Ukraine) Statut familial : Marié et 2 enfants Etudes : Doctorat en médecine obtenu à la Sergei M. Kirov Military Medicine Academy Statut professionnel: Colonel, Russian Air Force et travaille au centre d’entraînement des cosmonautes, le TsPK Roskosmos : Sélectionné le 09/02/1996 (RKKE-12) Précédents vols : - Clayton Conrad ANDERSON (Ingénieur de vol ISS) Lieu et date de naissance : 23/02/1959 ; Omaha (Nebraska) Statut familial : Marié et 2 enfants Etudes : Promu bachelier en physique à Hastings College, maîtrise en ingénierie aérospatiale à la Iowa State University Statut professionnel: Directeur du centre des opérations de secours à la Nasa Nasa : Sélectionné le 04/06/1998 (Groupe) Précédents vols : - Equipage Expedition 16 / Soyuz TM-11 Peggy A. -

Endeavour Set to Leave International Space Station Today 24 March 2008
Endeavour Set to Leave International Space Station Today 24 March 2008 who replaced European Space Agency astronaut Léopold Eyharts on the station. Eyharts is returning to Earth aboard Endeavour. The astronauts also performed five spacewalks while on the station. Endeavour is scheduled to land at Kennedy Space Center, Fla., Wednesday. Source: NASA STS-123 Mission Specialist Léopold Eyharts, pictured in the foreground, and Pilot Gregory H. Johnson work at the robotics station in the International Space Station's U.S. laboratory, Destiny. Credit: NASA The crew of space shuttle Endeavour is slated to leave the International Space Station today. The STS-123 and Expedition 16 crews will bid one another farewell, and the hatches between the two spacecraft will close at 5:13 p.m. EDT. Endeavour is scheduled to undock from the International Space Station at 7:56 p.m., ending its 12-day stay at the orbital outpost. STS-123 arrived at the station March 12, delivering the Japanese Logistics Module - Pressurized Section, the first pressurized component of the Japan Aerospace Exploration Agency’s Kibo laboratory, to the station. The crew of Endeavour also delivered the final element of the station’s Mobile Servicing System, the Canadian-built Dextre, also known as the Special Purpose Dextrous Manipulator. In addition, the STS-123 astronauts delivered Expedition 16 Flight Engineer Garrett Reisman, 1 / 2 APA citation: Endeavour Set to Leave International Space Station Today (2008, March 24) retrieved 24 September 2021 from https://phys.org/news/2008-03-endeavour-international-space-station-today.html This document is subject to copyright. -

Mir-125 in Normal and Malignant Hematopoiesis
Leukemia (2012) 26, 2011–2018 & 2012 Macmillan Publishers Limited All rights reserved 0887-6924/12 www.nature.com/leu SPOTLIGHT REVIEW MiR-125 in normal and malignant hematopoiesis L Shaham1,2, V Binder3,4,NGefen1,5, A Borkhardt3 and S Izraeli1,5 MiR-125 is a highly conserved microRNA throughout many different species from nematode to humans. In humans, there are three homologs (hsa-miR-125b-1, hsa-miR-125b-2 and hsa-miR-125a). Here we review a recent research on the role of miR-125 in normal and malignant hematopoietic cells. Its high expression in hematopoietic stem cells (HSCs) enhances self-renewal and survival. Its expression in specific subtypes of myeloid and lymphoid leukemias provides resistance to apoptosis and blocks further differentiation. A direct oncogenic role in the hematopoietic system has recently been demonstrated by several mouse models. Targets of miR-125b include key proteins regulating apoptosis, innate immunity, inflammation and hematopoietic differentiation. Leukemia (2012) 26, 2011–2018; doi:10.1038/leu.2012.90 Keywords: microRNA; hematopoiesis; hematological malignancies; acute myeloid leukemia; acute lymphoblastic leukemia MicroRNAs (miRNAs) are 21–23-nucleotide non-coding RNAs that nucleotides with the seed region of miR-125b (ebv-miR-BART21-5p, have crucial roles in fundamental biological processes by ebv-miR-BART8 and rlcv-miR-rL1-25). In humans, as in most of the regulating the levels of multiple proteins. They are transcribed genomes, there are two paralogs (hsa-miR-125b-1 on chromosome as primary miRNAs and processed in the nucleus by the RNase III 11 and hsa-miR-125b-2 on chromosome 21), coding for the same endonuclease DROSHA to liberate 70-nucleotide stem loops, the mature sequence. -

Expedition 16 Adding International Science
EXPEDITION 16 ADDING INTERNATIONAL SCIENCE The most complex phase of assembly since the NASA Astronaut Peggy Whitson, the fi rst woman Two days after launch, International Space Station was fi rst occupied seven commander of the ISS, and Russian Cosmonaut the Soyuz docked The International Space Station is seen by the crew of STS-118 years ago began when the Expedition 16 crew arrived Yuri Malenchenko were launched aboard the Soyuz to the Space Station as Space Shuttle Endeavour moves away. at the orbiting outpost. During this ambitious six-month TMA-11 spacecraft from the Baikonur Cosmodrome joining Expedition 15 endeavor, an unprecedented three Space Shuttle in Kazakhstan on October 10. The two veterans of Commander Fyodor crews will visit the Station delivering critical new earlier missions aboard the ISS were accompanied by Yurchikhin, Oleg Kotov, components – the American-built “Harmony” node, the Dr. Sheikh Muzaphar Shukor, an orthopedic surgeon both of Russia, and European Space Agency’s “Columbus” laboratory and and the fi rst Malaysian to fl y in space. NASA Flight Engineer Japanese “Kibo” element. Clayton Anderson. Shukor spent nine days CREW PROFILE on the ISS, returning to Earth in the Soyuz Peggy Whitson (Ph. D.) TMA-10 on October Expedition 16 Commander 21 with Yurchikhin and Born: February 9, 1960, Mount Ayr, Iowa Kotov who had been Education: Graduated with a bachelors degree in biology/chemistry from Iowa aboard the station since Wesleyan College, 1981 & a doctorate in biochemistry from Rice University, 1985 April 9. Experience: Selected as an astronaut in 1996, Whitson served as a Science Offi cer during Expedition 5. -

Orbiter Processing Facility
National Aeronautics and Space Administration Space Shuttle: Orbiter Processing From Landing To Launch he work of preparing a space shuttle for the same facilities. Inside is a description of an flight takes place primarily at the Launch orbiter processing flow; in this case, Discovery. Complex 39 Area. TThe process actually begins at the end of each acts Shuttle Landing Facility flight, with a landing at the center or, after landing At the end of its mission, the Space Shuttle f at an alternate site, the return of the orbiter atop a Discovery lands at the Shuttle Landing Facility on shuttle carrier aircraft. Kennedy’s Shuttle Landing one of two runway headings – Runway 15 extends Facility is the primary landing site. from the northwest to the southeast, and Runway There are now three orbiters in the shuttle 33 extends from the southeast to the northwest fleet: Discovery, Atlantis and Endeavour. Chal- – based on wind currents. lenger was destroyed in an accident in January After touchdown and wheelstop, the orbiter 1986. Columbia was lost during approach to land- convoy is deployed to the runway. The convoy ing in February 2003. consists of about 25 specially designed vehicles or Each orbiter is processed independently using units and a team of about 150 trained personnel, NASA some of whom assist the crew in disembarking from the orbiter. the orbiter and a “white room” is mated to the orbiter hatch. The The others quickly begin the processes necessary to “safe” the hatch is opened and a physician performs a brief preliminary orbiter and prepare it for towing to the Orbiter Processing Fa- medical examination of the crew members before they leave the cility. -

STS-132 Mission Summary
NASA Mission Summary National Aeronautics and Space Administration Washington, D.C. 20546 (202) 358-1100 STS-132 MISSION SUMMARY May 2010 SPACE SHUTTLE ATLANTIS Atlantis’ 12-day mission will deliver the Russian-built Mini Research Module-1 that will provide additional storage space and a new docking port for Russian Soyuz and Progress spacecraft. MRM-1, also known as Rassvet, which means dawn in Russian, will be permanently attached to the bottom port of the station’s Zarya module. MRM-1 will carry important hardware on its exterior including a radiator, airlock and a European robotic arm. Atlantis also will deliver addi- tional station hardware stored inside a cargo carrier. Three spacewalks are planned to stage spare components outside the station, including six spare batteries, a Ku-band antenna and spare parts for the Canadian Dextre robotic arm. Shuttle mission STS-132 is the final sched- uled flight for Atlantis . CREW Ken Ham Tony Antonelli (an-tuh-NEL-lee) Commander (Captain, U.S. Navy) Pilot (Commander, U.S. Navy) ● Veteran of one spaceflight, STS-124 pilot ● Veteran of one spaceflight, STS-119 pilot ● Age: 45, Born: Plainfield, N.J. ● Born: Detroit ● Married with two children ● Married with two children ● Logged 5,000+ hours in 40 different aircraft ● Logged 3,200+ hours in 41 different aircraft ● Call sign: Hock ● Interests include snow boarding and NASCAR Garrett Reisman (REESE-man) Michael Good Mission Specialist-1 Mission Specialist-2 (Col., U.S. Air Force, Ret.) ● Veteran flight engineer on Expedition 16 & 17 ● Veteran of one spaceflight, STS-125 ● Launched on STS-123; returned STS-124 ● Age: 47, Hometown: Broadview Heights, Ohio ● Age: 42, Hometown: Parsippany, N.J. -

See How the Flow
Integrated Defense Systems BOEING FRONTIERS s ewith- o in that re- g gion are a man- ageable 2,000 to 3,000 owdegrees, while just a few inches fl away from the orbiter’s surface the full e signed a force of heating results in a total tempera- h ture of up to 10,000 degrees. t special 6-inch-by- w As long as the orbiter’s surface is ho 6-inch (15.2-centemeter-by-15.2- e smooth, the boundary layer keeps the tiles’ Se centimeter) test tile, to be installed on the lower side of the orbiter port-side wing temperature within the limits of their de- Test tile to measure near the main landing gear door. The tile sign. But any interruption in the air fl ow will test airfl ow on three upcoming shut- causes a boundary layer “trip,” where tur- shuttle’s re-entry airfl ow tle fl ights, beginning with the STS-119 bulence behind the trip point brings down Discovery fl ight this December. The goal to the surface of the shuttle the extreme BY ED MEMI is to understand boundary layer transi- heat that was outside the laminar boundary layer. This could cause the tiles to overheat hen the Space Shuttle re-enters tions, and the data from this experiment will help NASA in its efforts to develop and damage the underlying surface. Earth’s atmosphere at 25 times The phenomenon is similar to a smooth the speed of sound, its Thermal new spacecraft such as the Orion crew ex- W ploration vehicle. -

+ STS-123 Press
CONTENTS Section Page STS-123 MISSION OVERVIEW................................................................................................ 1 TIMELINE OVERVIEW.............................................................................................................. 11 MISSION PROFILE................................................................................................................... 15 MISSION PRIORITIES............................................................................................................. 17 MISSION PERSONNEL............................................................................................................. 19 STS-123 ENDEAVOUR CREW .................................................................................................. 21 PAYLOAD OVERVIEW .............................................................................................................. 31 KIBO OVERVIEW.................................................................................................................................. 31 KIBO MISSION CONTROL CENTER ....................................................................................................... 39 TSUKUBA SPACE CENTER.................................................................................................................... 43 SPACE STATION INTEGRATION AND PROMOTION CENTER .................................................................. 47 JAXA’S EXPERIMENTS DURING THE 1J/A STAGE................................................................................. -

Spaceport News John F
June 10, 2011 Vol. 51, No. 11 Spaceport News John F. Kennedy Space Center - America’s gateway to the universe NASA/Sandra Joseph - Kevin O’Connell Endeavour ends STS-134, final mission Xenon lights help lead space shuttle Endeavour home on June 1. Endeavour landed for the final time on the Shuttle Landing Facility’s Runway 15, marking the 25th night landing of NASA’s Space Shuttle Pro- gram. Main gear touchdown was at 2:34:51 a.m. EDT, followed by nose gear touchdown at 2:35:04 a.m., and wheelstop at 2:35:36 a.m. STS-134 was the 25th and final flight for Endeavour, which has spent 299 days in space, orbited Earth 4,671 times and traveled 122,883,151 miles. Clickhere to take a look at Endeavour’s Click here to find out everything you Click here to get a complete Click here to view STS-134 images, Click here to watch the YouTube video Fact Sheet, including every mission’s need to know about the legacy of STS-134 mission overview and mission watch the mission’s video and listen to of Endeavour’s final landing with com- facts and figures. Endeavour. timeline. the mission’s audio. mentary. Optimus Prime award STS-135 Payload Final Shuttle Rollout Be prepared Inside this issue ... Page 2 Page 3 Page 6 Page 7 Page 2 SPACEPORT NEWS June 10, 2011 NASA transforms way students learn about technology By Rebecca Regan Spaceport News ptimus Prime once said, “There’s a thin line between Obeing a hero and being a memory.” Four fifth-graders from Union Park Elementary School in Orlando, Fla., recently became more than just a memory . -
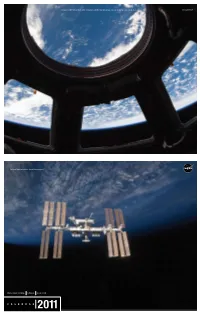
C a L E N D a R International Space Station
For more information on the International Space Station, visit: www.nasa.gov/station visit: Station, Space International the on information more For www.nasa.gov National Aeronautics and Space Administration INTERNATIONAL SPACE STATION CALENDAR 2011 A MESSAGE FROM THE PROGRAM MANAGER The International Space Station (ISS) is one of the greatest technological, geopolitical and engineering accomplishments in human 2011 history. The completion of the ISS on-orbit assembly allows for a focus on the multifaceted purpose of the ISS, one of scientific research, technology development, exploration and education. As a National Laboratory, the ISS will provide opportunities beyond NASA to academia, commercial entities and other government agencies to pursue their research and development needs in science, technology development and education. With everyone working together, we look forward to extending human presence beyond and improving life here on Earth. This calendar is designed to show all facets of the ISS using displays of astounding imagery and providing significant historical events with the hope of inspiring the next generation. NASA is appreciative of the commitment that America’s educators demonstrate each and every day as they instruct and shape the young students who will be tomorrow’s explorers and leaders. I hope you enjoy the calendar and are encouraged to learn new and exciting aspects about NASA and the ISS throughout the year. Regards, MICHAEL T. SUFFREDINI ISS Program Manager 1 2 2 3 4 6 5 LOOK HOW FAR WE’VE COME 20 JANUARY NASA has powered us into the 21st century through signature 11 accomplishments that are enduring icons of human achievement. -

STS-132 Press Kit Cover.Indd
National Aeronautics and Space Administration SPACE SHUTTLE MISSION STS-132 Finishing Touches PRESS KIT/May 2010 www.nasa.gov CONTENTS Section Page STS-132/ULF4 MISSION OVERVIEW ...................................................................................... 1 STS-132 TIMELINE OVERVIEW ............................................................................................... 13 MISSION PROFILE ................................................................................................................... 17 MISSION OBJECTIVES ............................................................................................................ 19 MISSION PERSONNEL ............................................................................................................. 23 STS-132 CREW ....................................................................................................................... 25 PAYLOAD OVERVIEW .............................................................................................................. 33 INTEGRATED CARGO CARRIER VERTICAL LIGHT DEPLOY (ICC-VLD) ................................................... 33 MINI-RESEARCH MODULE-1................................................................................................................. 36 RENDEZVOUS & DOCKING ....................................................................................................... 39 UNDOCKING, SEPARATION AND DEPARTURE ....................................................................................... 40 -

Apollo-Soyuz Test Project
--.I m ...ir,,.= The document_-contains materials on the Soyuz-Apollo test and consists of two parts, prepared by the USSR and USA sides res- pectively. Both parts outline the purposes and program of the mission, the spacecraft design, the flight plan and information on Joint and unilateral scientific experiments. Brief biographies of the cosmonauts and astronauts, the Joint mission crew members_ are also presented. The document covers technical support activities providing mission control and gives information about the ASTP Soviet and American leaders. As the USSR and USA parts of the document have been prepared independently, there might be duplication in the sections dealing with the Joint activities. The document is intended for press representatives and various mass information means. CONTENTS Page I.0 INTRODUCTION ....................................... 10 1.1 Background ......................................... I0 1,2 Apollo-Soyuz joint test project objectives .......... 13 2.0 COMPATIBILITY PROBLEMS ................... ......... • 15 2.1 Spacecraft compatibility conditions and principal solutions accepted for Apollo-Ssyuz Test Mission .... 15 2.2 Compatibility of ground flight control personnel ... 18 2_3 Methodological compatibility ....................... 20 3.0 SOYUZ SPACECRAFT ................................... 22 3.1 PurPose. Brief data on Soyuz spacecraft flights .... 22 3.2 Soyuz spacecraft description ....................... 25 3.2.1 General description of the Soyuz spacecraft.. 25 Main characteristics ........................