The End Protection Problem—An Unexpected Twist in the Tail
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Introduction of Human Telomerase Reverse Transcriptase to Normal Human Fibroblasts Enhances DNA Repair Capacity
Vol. 10, 2551–2560, April 1, 2004 Clinical Cancer Research 2551 Introduction of Human Telomerase Reverse Transcriptase to Normal Human Fibroblasts Enhances DNA Repair Capacity Ki-Hyuk Shin,1 Mo K. Kang,1 Erica Dicterow,1 INTRODUCTION Ayako Kameta,1 Marcel A. Baluda,1 and Telomerase, which consists of the catalytic protein subunit, No-Hee Park1,2 human telomerase reverse transcriptase (hTERT), the RNA component of telomerase (hTR), and several associated pro- 1School of Dentistry and 2Jonsson Comprehensive Cancer Center, University of California, Los Angeles, California teins, has been primarily associated with maintaining the integ- rity of cellular DNA telomeres in normal cells (1, 2). Telomer- ase activity is correlated with the expression of hTERT, but not ABSTRACT with that of hTR (3, 4). Purpose: From numerous reports on proteins involved The involvement of DNA repair proteins in telomere main- in DNA repair and telomere maintenance that physically tenance has been well documented (5–8). In eukaryotic cells, associate with human telomerase reverse transcriptase nonhomologous end-joining requires a DNA ligase and the (hTERT), we inferred that hTERT/telomerase might play a DNA-activated protein kinase, which is recruited to the DNA role in DNA repair. We investigated this possibility in nor- ends by the DNA-binding protein Ku. Ku binds to hTERT mal human oral fibroblasts (NHOF) with and without ec- without the need for telomeric DNA or hTR (9), binds the topic expression of hTERT/telomerase. telomere repeat-binding proteins TRF1 (10) and TRF2 (11), and Experimental Design: To study the effect of hTERT/ is thought to regulate the access of telomerase to telomere DNA telomerase on DNA repair, we examined the mutation fre- ends (12, 13). -

The Functions of DNA Damage Factor RNF8 in the Pathogenesis And
Int. J. Biol. Sci. 2019, Vol. 15 909 Ivyspring International Publisher International Journal of Biological Sciences 2019; 15(5): 909-918. doi: 10.7150/ijbs.31972 Review The Functions of DNA Damage Factor RNF8 in the Pathogenesis and Progression of Cancer Tingting Zhou 1, Fei Yi 1, Zhuo Wang 1, Qiqiang Guo 1, Jingwei Liu 1, Ning Bai 1, Xiaoman Li 1, Xiang Dong 1, Ling Ren 2, Liu Cao 1, Xiaoyu Song 1 1. Institute of Translational Medicine, China Medical University; Key Laboratory of Medical Cell Biology, Ministry of Education; Liaoning Province Collaborative Innovation Center of Aging Related Disease Diagnosis and Treatment and Prevention, Shenyang, Liaoning Province, China 2. Department of Anus and Intestine Surgery, First Affiliated Hospital of China Medical University, Shenyang, Liaoning Province, China Corresponding authors: Xiaoyu Song, e-mail: [email protected] and Liu Cao, e-mail: [email protected]. Key Laboratory of Medical Cell Biology, Ministry of Education; Institute of Translational Medicine, China Medical University; Collaborative Innovation Center of Aging Related Disease Diagnosis and Treatment and Prevention, Shenyang, Liaoning Province, 110122, China. Tel: +86 24 31939636, Fax: +86 24 31939636. © Ivyspring International Publisher. This is an open access article distributed under the terms of the Creative Commons Attribution (CC BY-NC) license (https://creativecommons.org/licenses/by-nc/4.0/). See http://ivyspring.com/terms for full terms and conditions. Received: 2018.12.03; Accepted: 2019.02.08; Published: 2019.03.09 Abstract The really interesting new gene (RING) finger protein 8 (RNF8) is a central factor in DNA double strand break (DSB) signal transduction. -

Novel Roles of Replication Protein a Phosphorylation in Cellular Response to DNA Damage Moises A
East Tennessee State University Digital Commons @ East Tennessee State University Electronic Theses and Dissertations Student Works 8-2013 Novel Roles of Replication Protein A Phosphorylation in Cellular Response to DNA Damage Moises A. Serrano East Tennessee State University Follow this and additional works at: https://dc.etsu.edu/etd Part of the Biochemistry, Biophysics, and Structural Biology Commons, and the Laboratory and Basic Science Research Commons Recommended Citation Serrano, Moises A., "Novel Roles of Replication Protein A Phosphorylation in Cellular Response to DNA Damage" (2013). Electronic Theses and Dissertations. Paper 1206. https://dc.etsu.edu/etd/1206 This Dissertation - Open Access is brought to you for free and open access by the Student Works at Digital Commons @ East Tennessee State University. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of Digital Commons @ East Tennessee State University. For more information, please contact [email protected]. Novel Roles of Replication Protein A Phosphorylation in the Cellular Response to DNA Damage _____________________________ A dissertation presented to the faculty of the Department of Biomedical Science East Tennessee State University In partial fulfillment of the requirements for the degree Doctor of Philosophy in Biomedical Science _____________________________ by Moises Alejandro Serrano August 2013 _____________________________ Yue Zou, Ph.D., Chair Phillip R. Musich, Ph.D. Antonio E. Rusiñol, Ph.D. Michelle M. Duffourc, Ph.D. William L. Stone, Ph.D. Keywords: DNA Repair, DNA Damage Responses, RPA, p53, Apoptosis ABSTRACT Novel Roles of Replication Protein A Phosphorylation in Cellular Response to DNA Damage by Moises Alejandro Serrano Human replication protein A (RPA) is an eukaryotic single-stranded DNA binding protein directly involved in a variety of DNA metabolic pathways including replication, recombination, DNA damage checkpoints and signaling, as well as all DNA repair pathways. -

Regulates Cellular Telomerase Activity by Methylation of TERT Promoter
www.impactjournals.com/oncotarget/ Oncotarget, 2017, Vol. 8, (No. 5), pp: 7977-7988 Research Paper Tianshengyuan-1 (TSY-1) regulates cellular Telomerase activity by methylation of TERT promoter Weibo Yu1, Xiaotian Qin2, Yusheng Jin1, Yawei Li2, Chintda Santiskulvong3, Victor Vu1, Gang Zeng4,5, Zuofeng Zhang6, Michelle Chow1, Jianyu Rao1,5 1Department of Pathology and Laboratory Medicine, David Geffen School of Medicine, University of California at Los Angeles, Los Angeles, CA, USA 2Beijing Boyuantaihe Biological Technology Co., Ltd., Beijing, China 3Genomics Core, Cedars-Sinai Medical Center, Los Angeles, CA, USA 4Department of Urology, David Geffen School of Medicine, University of California at Los Angeles, Los Angeles, CA, USA 5Jonsson Comprehensive Cancer Center, University of California at Los Angeles, Los Angeles, CA, USA 6Department of Epidemiology, School of Public Health, University of California at Los Angeles, Los Angeles, CA, USA Correspondence to: Jianyu Rao, email: [email protected] Keywords: TSY-1, hematopoietic cells, Telomerase, TERT, methylation Received: September 08, 2016 Accepted: November 24, 2016 Published: December 15, 2016 ABSTRACT Telomere and Telomerase have recently been explored as anti-aging and anti- cancer drug targets with only limited success. Previously we showed that the Chinese herbal medicine Tianshengyuan-1 (TSY-1), an agent used to treat bone marrow deficiency, has a profound effect on stimulating Telomerase activity in hematopoietic cells. Here, the mechanism of TSY-1 on cellular Telomerase activity was further investigated using HL60, a promyelocytic leukemia cell line, normal peripheral blood mononuclear cells, and CD34+ hematopoietic stem cells derived from umbilical cord blood. TSY-1 increases Telomerase activity in normal peripheral blood mononuclear cells and CD34+ hematopoietic stem cells with innately low Telomerase activity but decreases Telomerase activity in HL60 cells with high intrinsic Telomerase activity, both in a dose-response manner. -
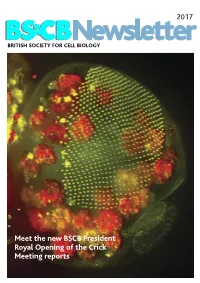
BSCB Newsletter 2017D
2017 BSCB Newsletter BRITISH SOCIETY FOR CELL BIOLOGY Meet the new BSCB President Royal Opening of the Crick Meeting reports 2017 CONTENTS BSCB Newsletter News 2 Book reviews 7 Features 8 Meeting Reports 24 Summer students 30 Society Business 33 Editorial Welcome to the 2017 BSCB newsletter. After several meeting hosted several well received events for our Front cover: years of excellent service, Kate Nobes has stepped PhD and Postdoc members, which we discuss on The head of a Drosophila pupa. The developing down and handed the reins over to me. I’ve enjoyed page 5. Our PhD and Postdoc reps are working hard compound eye (green) is putting together this years’ newsletter. It’s been great to make the event bigger and better for next year! The composed of several hundred simple units called ommatidia to hear what our members have been up to, and I social events were well attended including the now arranged in an extremely hope you will enjoy reading it. infamous annual “Pub Quiz” and disco after the regular array. The giant conference dinner. Members will be relieved to know polyploidy cells of the fat body (red), the fly equivalent of the The 2016 BSCB/DB spring meeting, organised by our we aren’t including any photos from that here. mammalian liver and adipose committee members Buzz Baum (UCL), Silke tissue, occupy a big area of the Robatzek and Steve Royle, had a particular focus on In this issue, we highlight the great work the BSCB head. Cells and Tissue Architecture, Growth & Cell Division, has been doing to engage young scientists. -

2. the MRN Complex
reviewreview The MRN complex: coordinating and mediating the response to broken chromosomes Michael van den Bosch, Ronan T.Bree & Noel F. Lowndes+ Genome Stability Laboratory, National University of Ireland, Galway, Ireland The MRE11–RAD50–NBS1 (MRN) protein complex has been linked mechanisms by modification and activation of specific repair factors. to many DNA metabolic events that involve DNA double-stranded Alternatively, if the damage is irreparable or an excessive number of breaks (DSBs). In vertebrate cells, all three components are encoded lesions is present, checkpoint signalling is also required to induce by essential genes, and hypomorphic mutations in any of the human apoptotic cell death. The two main mechanisms of DSB repair are genes can result in genome-instability syndromes. MRN is one of the non-homologous end joining (NHEJ) and homologous recombination first factors to be localized to the DNA lesion, where it might initially (HR; Barnes, 2001; van den Bosch et al., 2002). The malfunction have a structural role by tethering together, and therefore stabiliz- of these mechanisms can result in the fusion of DNA ends that were ing, broken chromosomes. This suggests that MRN could function as originally distant from one another in the genome, which generates a lesion-specific sensor. As well as binding to DNA, MRN has other chromosomal rearrangements such as inversions, translocations and roles in both the processing and assembly of large macromolecular deletions. The resulting disruption of gene expression can perturb complexes (known as foci) that facilitate efficient DSB responses. normal cell proliferation or result in cell death. Recently, a novel mediator protein, mediator of DNA damage checkpoint protein 1 (MDC1), was shown to co-immunoprecipitate The MRN complex and the repair of DNA damage with the MRN complex and regulate MRE11 foci formation. -

BASC, a Super Complex of BRCA1-Associated Proteins Involved in the Recognition and Repair of Aberrant DNA Structures
Downloaded from genesdev.cshlp.org on September 25, 2021 - Published by Cold Spring Harbor Laboratory Press BASC, a super complex of BRCA1-associated proteins involved in the recognition and repair of aberrant DNA structures Yi Wang,1,2,6 David Cortez,1,3,6 Parvin Yazdi,1,2 Norma Neff,5 Stephen J. Elledge,1,3,4 and Jun Qin1,2,7 1Verna and Mars McLean Department of Biochemistry and Molecular Biology, 2Department of Cellular and Molecular Biology, 3Howard Hughes Medical Institute, and 4Department of Molecular and Human Genetics, Baylor College of Medicine, Houston, Texas 77030 USA; 5Laboratory of Molecular Genetics, New York Blood Center, New York, New York 10021 USA We report the identities of the members of a group of proteins that associate with BRCA1 to form a large complex that we have named BASC (BRCA1-associated genome surveillance complex). This complex includes tumor suppressors and DNA damage repair proteins MSH2, MSH6, MLH1, ATM, BLM, and the RAD50–MRE11–NBS1 protein complex. In addition, DNA replication factor C (RFC), a protein complex that facilitates the loading of PCNA onto DNA, is also part of BASC. We find that BRCA1, the BLM helicase, and the RAD50–MRE11–NBS1 complex colocalize to large nuclear foci that contain PCNA when cells are treated with agents that interfere with DNA synthesis. The association of BRCA1 with MSH2 and MSH6, which are required for transcription-coupled repair, provides a possible explanation for the role of BRCA1 in this pathway. Strikingly, all members of this complex have roles in recognition of abnormal DNA structures or damaged DNA, suggesting that BASC may serve as a sensor for DNA damage. -

NBN Gene Analysis and It's Impact on Breast Cancer
Journal of Medical Systems (2019) 43: 270 https://doi.org/10.1007/s10916-019-1328-z IMAGE & SIGNAL PROCESSING NBN Gene Analysis and it’s Impact on Breast Cancer P. Nithya1 & A. ChandraSekar1 Received: 8 March 2019 /Accepted: 7 May 2019 /Published online: 5 July 2019 # Springer Science+Business Media, LLC, part of Springer Nature 2019 Abstract Single Nucleotide Polymorphism (SNP) researches have become essential in finding out the congenital relationship of structural deviations with quantitative traits, heritable diseases and physical responsiveness to different medicines. NBN is a protein coding gene (Breast Cancer); Nibrin is used to fix and rebuild the body from damages caused because of strand breaks (both singular and double) associated with protein nibrin. NBN gene was retrieved from dbSNP/NCBI database and investigated using computational SNP analysis tools. The encrypted region in SNPs (exonal SNPs) were analyzed using software tools, SIFT, Provean, Polyphen, INPS, SNAP and Phd-SNP. The 3’ends of SNPs in un-translated region were also investigated to determine the impact of binding. The association of NBN gene polymorphism leads to several diseases was studied. Four SNPs were predicted to be highly damaged in coding regions which are responsible for the diseases such as, Aplastic Anemia, Nijmegan breakage syndrome, Microsephaly normal intelligence, immune deficiency and hereditary cancer predisposing syndrome (clivar). The present study will be helpful in finding the suitable drugs in future for various diseases especially for breast cancer. Keywords NBN . Single nucleotide polymorphism . Double strand breaks . nsSNP . Associated diseases Introduction NBN has a more complex structure due to its interaction with large proteins formed from the ATM gene which is NBN (Nibrin) is a protein coding gene, it is also known as highly essential in identifying damaged strands of DNA NBS1, Cell cycle regulatory Protein P95, is situated on and facilitating their repair [1]. -

Genetic and Genomic Analysis of Hyperlipidemia, Obesity and Diabetes Using (C57BL/6J × TALLYHO/Jngj) F2 Mice
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Nutrition Publications and Other Works Nutrition 12-19-2010 Genetic and genomic analysis of hyperlipidemia, obesity and diabetes using (C57BL/6J × TALLYHO/JngJ) F2 mice Taryn P. Stewart Marshall University Hyoung Y. Kim University of Tennessee - Knoxville, [email protected] Arnold M. Saxton University of Tennessee - Knoxville, [email protected] Jung H. Kim Marshall University Follow this and additional works at: https://trace.tennessee.edu/utk_nutrpubs Part of the Animal Sciences Commons, and the Nutrition Commons Recommended Citation BMC Genomics 2010, 11:713 doi:10.1186/1471-2164-11-713 This Article is brought to you for free and open access by the Nutrition at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Nutrition Publications and Other Works by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. Stewart et al. BMC Genomics 2010, 11:713 http://www.biomedcentral.com/1471-2164/11/713 RESEARCH ARTICLE Open Access Genetic and genomic analysis of hyperlipidemia, obesity and diabetes using (C57BL/6J × TALLYHO/JngJ) F2 mice Taryn P Stewart1, Hyoung Yon Kim2, Arnold M Saxton3, Jung Han Kim1* Abstract Background: Type 2 diabetes (T2D) is the most common form of diabetes in humans and is closely associated with dyslipidemia and obesity that magnifies the mortality and morbidity related to T2D. The genetic contribution to human T2D and related metabolic disorders is evident, and mostly follows polygenic inheritance. The TALLYHO/ JngJ (TH) mice are a polygenic model for T2D characterized by obesity, hyperinsulinemia, impaired glucose uptake and tolerance, hyperlipidemia, and hyperglycemia. -

Identification of Conserved Genes Triggering Puberty in European Sea
Blázquez et al. BMC Genomics (2017) 18:441 DOI 10.1186/s12864-017-3823-2 RESEARCHARTICLE Open Access Identification of conserved genes triggering puberty in European sea bass males (Dicentrarchus labrax) by microarray expression profiling Mercedes Blázquez1,2* , Paula Medina1,2,3, Berta Crespo1,4, Ana Gómez1 and Silvia Zanuy1* Abstract Background: Spermatogenesisisacomplexprocesscharacterized by the activation and/or repression of a number of genes in a spatio-temporal manner. Pubertal development in males starts with the onset of the first spermatogenesis and implies the division of primary spermatogonia and their subsequent entry into meiosis. This study is aimed at the characterization of genes involved in the onset of puberty in European sea bass, and constitutes the first transcriptomic approach focused on meiosis in this species. Results: European sea bass testes collected at the onset of puberty (first successful reproduction) were grouped in stage I (resting stage), and stage II (proliferative stage). Transition from stage I to stage II was marked by an increase of 11ketotestosterone (11KT), the main fish androgen, whereas the transcriptomic study resulted in 315 genes differentially expressed between the two stages. The onset of puberty induced 1) an up-regulation of genes involved in cell proliferation, cell cycle and meiosis progression, 2) changes in genes related with reproduction and growth, and 3) a down-regulation of genes included in the retinoic acid (RA) signalling pathway. The analysis of GO-terms and biological pathways showed that cell cycle, cell division, cellular metabolic processes, and reproduction were affected, consistent with the early events that occur during the onset of puberty. -

CREB-Binding Protein Regulates Ku70 Acetylation in Response to Ionization Radiation in Neuroblastoma
Published OnlineFirst December 5, 2012; DOI: 10.1158/1541-7786.MCR-12-0065 Molecular Cancer DNA Damage and Repair Research CREB-Binding Protein Regulates Ku70 Acetylation in Response to Ionization Radiation in Neuroblastoma Chitra Subramanian1, Manila Hada2,3, Anthony W. Opipari Jr2, Valerie P. Castle1, and Roland P.S. Kwok2,3 Abstract Ku70 was originally described as an autoantigen, but it also functions as a DNA repair protein in the nucleus and as an antiapoptotic protein by binding to Bax in the cytoplasm, blocking Bax-mediated cell death. In neuroblastoma (NB) cells, Ku70's binding with Bax is regulated by Ku70 acetylation such that increasing Ku70 acetylation results in Bax release, triggering cell death. Although regulating cytoplasmic Ku70 acetylation is important for cell survival, the role of nuclear Ku70 acetylation in DNA repair is unclear. Here, we showed that Ku70 acetylation in the nucleus is regulated by the CREB-binding protein (CBP), and that Ku70 acetylation plays an important role in DNA repair in NB cells. We treated NB cells with ionization radiation and measured DNA repair activity as well as Ku70 acetylation status. Cytoplasmic and nuclear Ku70 were acetylated after ionization radiation in NB cells. Interestingly, cytoplasmic Ku70 was redistributed to the nucleus following irradiation. Depleting CBP in NB cells results in reducing Ku70 acetylation and enhancing DNA repair activity in NB cells, suggesting nuclear Ku70 acetylation may have an inhibitory role in DNA repair. These results provide support for the hypothesis that enhancing Ku70 acetylation, through deacetylase inhibition, may potentiate the effect of ionization radiation in NB cells. -

A Balanced Transcription Between Telomerase and the Telomeric DNA
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by HAL-ENS-LYON A balanced transcription between telomerase and the telomeric DNA-binding proteins TRF1, TRF2 and Pot1 in resting, activated, HTLV-1-transformed and Tax-expressing human T lymphocytes. Emmanuelle Escoffier, Am´elieRezza, Aude Roborel de Climens, Aur´elie Belleville, Louis Gazzolo, Eric Gilson, Madeleine Duc Dodon To cite this version: Emmanuelle Escoffier, Am´elieRezza, Aude Roborel de Climens, Aur´elieBelleville, Louis Gaz- zolo, et al.. A balanced transcription between telomerase and the telomeric DNA-binding proteins TRF1, TRF2 and Pot1 in resting, activated, HTLV-1-transformed and Tax-expressing human T lymphocytes.. Retrovirology, BioMed Central, 2005, 2, pp.77. <10.1186/1742-4690- 2-77>. <inserm-00089278> HAL Id: inserm-00089278 http://www.hal.inserm.fr/inserm-00089278 Submitted on 16 Aug 2006 HAL is a multi-disciplinary open access L'archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destin´eeau d´ep^otet `ala diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publi´esou non, lished or not. The documents may come from ´emanant des ´etablissements d'enseignement et de teaching and research institutions in France or recherche fran¸caisou ´etrangers,des laboratoires abroad, or from public or private research centers. publics ou priv´es. Retrovirology BioMed Central Research Open Access A balanced transcription between telomerase and the