Immuno-Oncology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Systemic Immunotherapy for Urothelial Cancer: Current Trends and Future Directions
Review Systemic Immunotherapy for Urothelial Cancer: Current Trends and Future Directions Shilpa Gupta 1,†, David Gill 2,†, Austin Poole 2 and Neeraj Agarwal 2,* 1 Masonic Cancer Center, University of Minnesota, Minneapolis, MN 55455, USA; [email protected] 2 Huntsman Cancer Institute, University of Utah, Salt Lake City, UT 84112, USA; [email protected] (D.G.); [email protected] (A.P.) * Correspondence: [email protected]; Tel.: +1-801-213-5658; Fax: +1-801-585-0124 † These authors contributed equally to this work. Academic Editor: Vita Golubovskaya Received: 14 December 2016; Accepted: 18 January 2017; Published: 27 January 2017 Abstract: Urothelial cancer of the bladder, renal pelvis, ureter, and other urinary organs is the fifth most common cancer in the United States, and systemic platinum-based chemotherapy remains the standard of care for first-line treatment of advanced/metastatic urothelial carcinoma (UC). Until recently, there were very limited options for patients who are refractory to chemotherapy, or do not tolerate chemotherapy due to toxicities and overall outcomes have remained very poor. While the role of immunotherapy was first established in non-muscle invasive bladder cancer in the 1970s, no systemic immunotherapy was approved for advanced disease until the recent approval of a programmed death ligand-1 (PD-L1) inhibitor, atezolizumab, in patients with advanced/metastatic UC who have progressed on platinum-containing regimens. This represents a significant milestone in this disease after a void of over 30 years. In addition to atezolizumab, a variety of checkpoint inhibitors have shown a significant activity in advanced/metastatic urothelial carcinoma and are expected to gain Food and Drug Administration (FDA) approval in the near future. -

IL-10 and ICOS Differentially Regulate T Cell Responses in the Brain During Chronic 2 Toxoplasma Gondii Infection 3
bioRxiv preprint doi: https://doi.org/10.1101/418665; this version posted September 15, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 IL-10 and ICOS differentially regulate T cell responses in the brain during chronic 2 Toxoplasma gondii infection 3 4 Carleigh A. O’Brien*, Samantha J. Batista*, Katherine M. Still*, and Tajie H. Harris* 5 6 Affiliations: *Center for Brain Immunology and Glia, Department of Neuroscience, University of 7 Virginia, Charlottesville, VA 22908 8 9 Corresponding Author: 10 Tajie H. Harris, MR-4 Room 6148, 409 Lane Road, Charlottesville, VA 22908 11 Phone: 434-982-6916 12 Fax: 434-9824380 13 Email: [email protected] 14 15 This work was funded by the National Institutes of Health grants R01NS091067 to T.H.H., 16 T32AI007496 to C.A.O, T32AI007046 to S.J.B., and T32GM008328 to K.M.S., as well as the 17 University of Virginia Robert R. Wagner Fellowship to C.A.O. 18 19 20 21 22 23 24 1 bioRxiv preprint doi: https://doi.org/10.1101/418665; this version posted September 15, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 25 Abstract 26 Control of chronic CNS infection with the parasite Toxoplasma gondii requires an ongoing T cell 27 response in the brain. Immunosuppressive cytokines are also important for preventing lethal 28 immunopathology during chronic infection. -

CD226 T Cells Expressing the Receptors TIGIT and Divergent Phenotypes of Human Regulatory
The Journal of Immunology Divergent Phenotypes of Human Regulatory T Cells Expressing the Receptors TIGIT and CD226 Christopher A. Fuhrman,*,1 Wen-I Yeh,*,1 Howard R. Seay,* Priya Saikumar Lakshmi,* Gaurav Chopra,† Lin Zhang,* Daniel J. Perry,* Stephanie A. McClymont,† Mahesh Yadav,† Maria-Cecilia Lopez,‡ Henry V. Baker,‡ Ying Zhang,x Yizheng Li,{ Maryann Whitley,{ David von Schack,x Mark A. Atkinson,* Jeffrey A. Bluestone,‡ and Todd M. Brusko* Regulatory T cells (Tregs) play a central role in counteracting inflammation and autoimmunity. A more complete understanding of cellular heterogeneity and the potential for lineage plasticity in human Treg subsets may identify markers of disease pathogenesis and facilitate the development of optimized cellular therapeutics. To better elucidate human Treg subsets, we conducted direct transcriptional profiling of CD4+FOXP3+Helios+ thymic-derived Tregs and CD4+FOXP3+Helios2 T cells, followed by comparison with CD4+FOXP32Helios2 T conventional cells. These analyses revealed that the coinhibitory receptor T cell Ig and ITIM domain (TIGIT) was highly expressed on thymic-derived Tregs. TIGIT and the costimulatory factor CD226 bind the common ligand CD155. Thus, we analyzed the cellular distribution and suppressive activity of isolated subsets of CD4+CD25+CD127lo/2 T cells expressing CD226 and/or TIGIT. We observed TIGIT is highly expressed and upregulated on Tregs after activation and in vitro expansion, and is associated with lineage stability and suppressive capacity. Conversely, the CD226+TIGIT2 population was associated with reduced Treg purity and suppressive capacity after expansion, along with a marked increase in IL-10 and effector cytokine production. These studies provide additional markers to delineate functionally distinct Treg subsets that may help direct cellular therapies and provide important phenotypic markers for assessing the role of Tregs in health and disease. -

Single-Cell RNA Sequencing Demonstrates the Molecular and Cellular Reprogramming of Metastatic Lung Adenocarcinoma
ARTICLE https://doi.org/10.1038/s41467-020-16164-1 OPEN Single-cell RNA sequencing demonstrates the molecular and cellular reprogramming of metastatic lung adenocarcinoma Nayoung Kim 1,2,3,13, Hong Kwan Kim4,13, Kyungjong Lee 5,13, Yourae Hong 1,6, Jong Ho Cho4, Jung Won Choi7, Jung-Il Lee7, Yeon-Lim Suh8,BoMiKu9, Hye Hyeon Eum 1,2,3, Soyean Choi 1, Yoon-La Choi6,10,11, Je-Gun Joung1, Woong-Yang Park 1,2,6, Hyun Ae Jung12, Jong-Mu Sun12, Se-Hoon Lee12, ✉ ✉ Jin Seok Ahn12, Keunchil Park12, Myung-Ju Ahn 12 & Hae-Ock Lee 1,2,3,6 1234567890():,; Advanced metastatic cancer poses utmost clinical challenges and may present molecular and cellular features distinct from an early-stage cancer. Herein, we present single-cell tran- scriptome profiling of metastatic lung adenocarcinoma, the most prevalent histological lung cancer type diagnosed at stage IV in over 40% of all cases. From 208,506 cells populating the normal tissues or early to metastatic stage cancer in 44 patients, we identify a cancer cell subtype deviating from the normal differentiation trajectory and dominating the metastatic stage. In all stages, the stromal and immune cell dynamics reveal ontological and functional changes that create a pro-tumoral and immunosuppressive microenvironment. Normal resident myeloid cell populations are gradually replaced with monocyte-derived macrophages and dendritic cells, along with T-cell exhaustion. This extensive single-cell analysis enhances our understanding of molecular and cellular dynamics in metastatic lung cancer and reveals potential diagnostic and therapeutic targets in cancer-microenvironment interactions. 1 Samsung Genome Institute, Samsung Medical Center, Seoul 06351, Korea. -

Certain Tadalafil Or Any Salt Solvate Thereof and Products Containing
In the Matter of Certain Tadalafil or any Salt or Solvate Thereof and Products Containing Same Investigation No. 337-TA-539 Publication 3992 May2008 U.S. International Trade Commission Washington, i::>C 20436 U.S. International Trade Commission COMMISSIONERS Daniel R. Pearson, Chairman Shara L. Aranoff, Vice Chairman Deanna Tanner Okun Charlotte R. Lane Irving A. Williamson* Dean A. Pinkert* *Coirmissioner Marcia E. Miller, whose term ended on September 6, 2005, participated in the decision to institute the investigation. Commissioner Shara L. Aranoff, whose term commenced on September 6, 2005, participated in all subsequent phases of the investigation. Commissioner Irving A. Williamson was sworn in on February 7, 2007, and Commissioner Dean A. Pinkert was sworn in on February 26, '2007; they did not participate in this investigation. Commissioner Stephen Koplan, whose term ended on February 6, 2007, and Commissioner Jennifer A. Hillman, whose term ended on February 23, 2007, did participate in this investigation. ' Address all communications to Secretary to the Commission United States International Trade Commission Washington, DC 20436 U.S. International Trade Commission Washington, DC 20436 www.usitc.gov In the Matter of Certain Tadalafil or any Salt or Solvate Thereof and Products Containing Same Investigation No. 337-TA-539 Publication 3992 May 2008 UNITED STATES INTERNATIONAL TRADE COMMISSION Washington, D.C. 20436 ) In the Matter of ) ) CERTAIN TADALAFIL OR ANY SALT OR ) Inv. No. 337-TA-539 SOLVATETHEREOFANDPRODUCTS ) CONTAINING SAME ) ~~~~~~~~~~~~~~~~~> NOTICE OF COMMISSION ISSUANCE OF GENERAL EXCLUSION ORDER; DECISION TO GRANT MOTION TO FILE A SURREPLY; TERMINATION OF THE INVESTIGATION AGENCY: U.S. International Trade Commission. -

Classification Decisions Taken by the Harmonized System Committee from the 47Th to 60Th Sessions (2011
CLASSIFICATION DECISIONS TAKEN BY THE HARMONIZED SYSTEM COMMITTEE FROM THE 47TH TO 60TH SESSIONS (2011 - 2018) WORLD CUSTOMS ORGANIZATION Rue du Marché 30 B-1210 Brussels Belgium November 2011 Copyright © 2011 World Customs Organization. All rights reserved. Requests and inquiries concerning translation, reproduction and adaptation rights should be addressed to [email protected]. D/2011/0448/25 The following list contains the classification decisions (other than those subject to a reservation) taken by the Harmonized System Committee ( 47th Session – March 2011) on specific products, together with their related Harmonized System code numbers and, in certain cases, the classification rationale. Advice Parties seeking to import or export merchandise covered by a decision are advised to verify the implementation of the decision by the importing or exporting country, as the case may be. HS codes Classification No Product description Classification considered rationale 1. Preparation, in the form of a powder, consisting of 92 % sugar, 6 % 2106.90 GRIs 1 and 6 black currant powder, anticaking agent, citric acid and black currant flavouring, put up for retail sale in 32-gram sachets, intended to be consumed as a beverage after mixing with hot water. 2. Vanutide cridificar (INN List 100). 3002.20 3. Certain INN products. Chapters 28, 29 (See “INN List 101” at the end of this publication.) and 30 4. Certain INN products. Chapters 13, 29 (See “INN List 102” at the end of this publication.) and 30 5. Certain INN products. Chapters 28, 29, (See “INN List 103” at the end of this publication.) 30, 35 and 39 6. Re-classification of INN products. -

Bristol-Myers Squibb 2019 Annual Report
We’re inspired by a single vision: 2019 ANNUAL REPORT Our Mission To discover, develop and deliver innovative medicines that help patients prevail over serious diseases Our Vision To be the world's leading biopharma company that transforms patients' lives through science The patient stories shared in this Annual Report depict individual patient responses to our medicines or investigational compounds and are not representative of all patient responses. In addition, there is no guarantee that potential drugs or indications still in development will receive regulatory approval. Bristol Myers Squibb 2019 Annual Report Myers Bristol Made Strong® is a registered mark of the Made Strong organization. Used with permission. Letter from the Chairman and CEO At Bristol Myers Squibb, we are inspired by our mission – to discover, develop and deliver innovative medicines that help patients prevail over serious diseases. Every day, we focus on innovations that drive meaningful breakthroughs so we can bring life-saving medicines to people around the world. 2019 was a transformative year for us. A BIOPHARMA LEADER THE POWER OF FOLLOWING THE SCIENCE The acquisition of Celgene Corporation significantly We start this next chapter at a time when unprecedented advanced our strategy to create a leading biopharma scientific breakthroughs are advancing the treatment company by bringing together the speed and agility of disease as never before. Bristol Myers Squibb is well of a biotech with the global scale and resources of an positioned in scientific hubs of innovation in the U.S. and established pharmaceutical company. Our new company globally, and we collaborate with a broad network of global has strong commercial franchises in oncology, hematology, partners across our disease areas of focus to sustain our immunology and cardiovascular disease, one of the most leadership and continue to transform patient outcomes. -

Binding Mode of the Side-By-Side Two-Igv Molecule CD226/DNAM-1 to Its Ligand CD155/Necl-5
Binding mode of the side-by-side two-IgV molecule CD226/DNAM-1 to its ligand CD155/Necl-5 Han Wanga, Jianxun Qib, Shuijun Zhangb,1, Yan Lib, Shuguang Tanb,2, and George F. Gaoa,b,2 aResearch Network of Immunity and Health (RNIH), Beijing Institutes of Life Science, Chinese Academy of Sciences (CAS), 100101 Beijing, China; and bCAS Key Laboratory of Pathogenic Microbiology and Immunology, Institute of Microbiology, Chinese Academy of Sciences, 100101 Beijing, China Edited by K. Christopher Garcia, Stanford University School of Medicine, Stanford, CA, and approved December 3, 2018 (received for review September 11, 2018) Natural killer (NK) cells are important component of innate immu- CD226, also known as DNAM-1, belongs to the Ig superfamily nity and also contribute to activating and reshaping the adaptive and contains two extracellular Ig-like domains (CD226-D1 and immune responses. The functions of NK cells are modulated by CD226-D2), and is widely expressed in monocytes, platelets, multiple inhibitory and stimulatory receptors. Among these recep- T cells, and most of the resting NK cells (8, 13, 19, 20). The tors, the activating receptor CD226 (DNAM-1) mediates NK cell intracellular domain of CD226 does not contain a tyrosine-based activation via binding to its nectin-like (Necl) family ligand, CD155 activation motif, which is accepted as responsible for activating (Necl-5). Here, we present a unique side-by-side arrangement signal transduction of stimulatory molecules (13). Instead, it pattern of two tandem immunoglobulin V-set (IgV) domains transmits the downstream signaling by phosphorylation of in- deriving from the ectodomains of both human CD226 (hCD226- tracellular phosphorylation sites and subsequent association with ecto) and mouse CD226 (mCD226-ecto), which is substantially integrin lymphocyte function-associated antigen 1 (21). -

WO 2016/106159 Al 30 June 2016 (30.06.2016) W P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/106159 Al 30 June 2016 (30.06.2016) W P O P C T (51) International Patent Classification: (74) Agents: BALICKY, Eric, M. et al; Hamilton, Brook, C07K 16/28 (2006.01) Smith & Reynolds, P.C., 530 Virginia Rd, P.O. Box 9133, Concord, MA 01742-9133 (US). (21) International Application Number: PCT/US20 15/066954 (81) Designated States (unless otherwise indicated, for every kind of national protection available): AE, AG, AL, AM, (22) Date: International Filing AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, 19 December 2015 (19. 12.2015) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, (25) Filing Language: English DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, (26) Publication Language: English KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, (30) Priority Data: MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, 62/095,675 22 December 2014 (22. 12.2014) US PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, 62/220,199 17 September 2015 (17.09.2015) US SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, 62/25 1,082 4 November 2015 (04. 11.2015) US TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. -
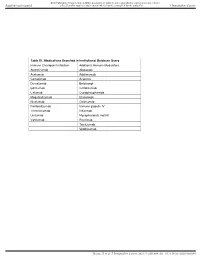
Table S1. Medications Searched in Institutional Database Query Immune Checkpoint Inhibitors Additional Immune Modulators Atezol
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) J Immunother Cancer Table S1. Medications Searched in Institutional Database Query Immune Checkpoint Inhibitors Additional Immune Modulators Atezolizumab Abatacept Avelumab Adalimumab Cemiplimab Anakinra Durvalumab Belatacept Ipilimumab Certolizumab Lirilumab Cyclophosphamide Mogamulizumab Etanercept Nivolumab Golimumab Pembrolizumab Immune globulin IV Tremelimumab Infliximab Urelumab Mycophenolate mofetil Varlilumab Rituximab Tocilizumab Vedolizumab Beattie J, et al. J Immunother Cancer 2021; 9:e001884. doi: 10.1136/jitc-2020-001884 BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) J Immunother Cancer Table S2. Toxicity of additional immune modulators Treatment detail Toxicity Patient 1 day 18,32 Infliximab dosed day 59 P.aeruginosa, S.marcescens pneumonia; died Patient 2 day 9 Infliximab dosed day 44 Febrile neutropenia; P. aeruginosa SBP and day 26 Mycophenolate initiated sepsis; C. albicans fungemia; treated and discharged Patient 8 day 4,11 Infliximab dosed day 14 Disseminated HSV-1; died Patient 26 day 79-128 Infliximab dosed (x7) day 130 E. faecalis, P. aeruginosa bacteremia; died; day 81, 97 Cyclophosphamide dosed Fungal pneumonia on autopsy Beattie J, et al. J Immunother Cancer 2021; 9:e001884. doi: 10.1136/jitc-2020-001884 BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) J Immunother Cancer Table S3. -

Cytokine Regulation in Human CD4 T Cells by the Aryl Hydrocarbon
www.nature.com/scientificreports OPEN Cytokine Regulation in Human CD4 T Cells by the Aryl Hydrocarbon Receptor and Gq-Coupled Received: 17 November 2017 Accepted: 9 July 2018 Receptors Published: xx xx xxxx Jeremy P. McAleer1, Jun Fan2, Bryanna Roar1, Donald A. Primerano2 & James Denvir2 Th17 cells contribute to host defense on mucosal surfaces but also provoke autoimmune diseases when directed against self-antigens. Identifying therapeutic targets that regulate Th17 cell diferentiation and/or cytokine production has considerable value. Here, we study the aryl hydrocarbon receptor (AhR)- dependent transcriptome in human CD4 T cells treated with Th17-inducing cytokines. We show that the AhR reciprocally regulates IL-17 and IL-22 production in human CD4 T cells. Global gene expression analysis revealed that AhR ligation decreased IL21 expression, correlating with delayed upregulation of RORC during culture with Th17-inducing cytokines. Several of the AhR-dependent genes have known roles in cellular assembly, organization, development, growth and proliferation. We further show that expression of GPR15, GPR55 and GPR68 positively correlates with IL-22 production in the presence of the AhR agonist FICZ. Activation of GPR68 with the lorazepam derivative ogerin resulted in suppression of IL-22 and IL-10 secretion by T cells, with no efect on IL-17. Under neutral Th0 conditions, ogerin and the Gq/11 receptor inhibitor YM254890 blunted IL-22 induction by FICZ. These data reveal the AhR- dependent transcriptome in human CD4 T cells and suggest the mechanism through which the AhR regulates T cell function may be partially dependent on Gq-coupled receptors including GPR68. -

Phosphodiesterase Type 5 Inhibitors for the Treatment of Erectile Dysfunction in Patients with Diabetes Mellitus
International Journal of Impotence Research (2002) 14, 466–471 ß 2002 Nature Publishing Group All rights reserved 0955-9930/02 $25.00 www.nature.com/ijir Phosphodiesterase type 5 inhibitors for the treatment of erectile dysfunction in patients with diabetes mellitus MA Vickers1,2* and R Satyanarayana1 1Department of Surgery, Togus VA Medical Center, Togus, Maine, USA; and 2Department of Surgery, Division of Urology, University of Massachusetts Medical School, Worcester, Massachusetts, USA Sildenafil, a phosphodiesterase 5 (PDE5) inhibitor, has become a first-line therapy for diabetic patients with erectile dysfunction (ED). The efficacy in this subgroup, based on the Global Efficacy Question, is 56% vs 84% in a selected group of non-diabetic men with ED. Two novel PDE5 inhibitors, tadalafil (Lilly ICOS) and vardenafil (Bayer), have recently completed efficacy and safety clinical trials in ‘general’ and diabetic study populations and are now candidates for US FDA approval. A summary analysis of the phase three clinical trials of sildenafil, tadalafil and vardenafil in both study populations is presented to provide a foundation on which the evaluation of the role of the individual PDE5 inhibitors for the treatment of patients with ED and DM can be built. International Journal of Impotence Research (2002) 14, 466–471. doi:10.1038=sj.ijir.3900910 Keywords: phosphodiesterase inhibitor; erectile dysfunction; diabetes mellitus; sildenafil; tadalafil; vardenafil Introduction (NO), vasointestinal peptide and prostacyclin, de- creases. Additionally, the endothelial cells that line the cavernosal arteries and sinusoids have a Pathophysiology of ED in diabetes decreased response to nitric oxide due to increased production of advanced glycation end-products and changes associated with insulin resistance.5,6 The Diabetes mellitus is a risk factor for erectile diabetic also experiences a decreased level of dysfunction (ED).