Investigation of an Invasive Crayfish and Its Relation to Two Imperiled Native Crayfishes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fisheries Across the Eastern Continental Divide
Fisheries Across the Eastern Continental Divide Abstracts for oral presentations and posters, 2010 Spring Meeting of the Southern Division of the American Fisheries Society Asheville, NC 1 Contributed Paper Oral Presentation Potential for trophic competition between introduced spotted bass and native shoal bass in the Flint River Sammons, S.M.*, Auburn University. Largemouth bass, shoal bass, and spotted bass were collected from six sites over four seasons on the Flint River, Georgia to assess food habits. Diets of all three species was very broad; 10 categories of invertebrates and 15 species of fish were identified from diets. Since few large spotted bass were collected, all comparisons among species were conducted only for juvenile fish (< 200 mm) and subadult fish (200-300 mm). Juvenile largemouth bass diets were dominated by fish in all seasons, mainly sunfish. Juvenile largemouth bass rarely ate insects except in spring, when all three species consumed large numbers of insects. In contrast, juvenile shoal bass diets were dominated by insects in all seasons but winter. Juvenile spotted bass diets were more varied- highly piscivorous in the fall and winter and highly insectivorous in spring and summer. Diets of subadult largemouth bass were similar to that of juvenile fish, and heavily dominated by fish, particularly sunfish. Similar to juveniles, diets of subadult shoal bass were much less piscivorous than largemouth bass. Crayfish were important components of subadult shoal bass diets in all seasons but summer. Insects were important components of shoal bass diets in fall and summer. Diets of subadult spotted bass were generally more piscivorous than shoal bass, but less than largemouth bass. -

The Evolution of Crayfishes of the Genus Orconectes Section Limosus (Crustacea: Decopoda)
THE OHIO JOURNAL OF SCIENCE Vol. 62 MARCH, 1962 No. 2 THE EVOLUTION OF CRAYFISHES OF THE GENUS ORCONECTES SECTION LIMOSUS (CRUSTACEA: DECOPODA) RENDELL RHOADES Department of Zoology and Entomology, The Ohio State University, Columbus 10 The earliest described crayfish species now included in the Section limosus of the Genus Orconectes was described by Samuel Constantine Rafinesque (1817: 42). He reported the species, which he named Astacus limosus, "in the muddy banks of the Delaware, near Philadelphia." How ironical it now seems, that when Rafinesque located at Transylvania three years later and traveled to Henderson, Kentucky, to visit a fellow naturalist, John J. Audubon, he could have collected from the streams of western Kentucky a crayfish that he might have identified as the species he had described from the Delaware. We now know that these streams of the knobstone and pennyroyal uplands are the home of parent stock of this group. Moreover, this parental population on the Cumberland Plateau is now separated from Rafinesque's Orconectes limosus of the Atlantic drainage by more than 500 miles of mountainous terrain. Even Rafinesque, with his flair for accuracy and vivid imagination, would have been taxed to explain this wide separation had he known it. A decade after the death of Rafinesque, Dr. W. T. Craige received a blind crayfish from Mammoth Cave. An announcement of the new crayfish, identi- fied as "Astacus bartonii (?)" appeared in the Proceedings of the Academy of Natural Science of Philadelphia (1842: 174-175). Within two years the impact of Dr. Craige's announcement was evidenced by numerous popular articles both here and abroad. -

Complaint for Declaratory and Injunctive Relief 1 1 2 3 4 5 6 7 8 9
1 Justin Augustine (CA Bar No. 235561) Jaclyn Lopez (CA Bar No. 258589) 2 Center for Biological Diversity 351 California Street, Suite 600 3 San Francisco, CA 94104 Tel: (415) 436-9682 4 Fax: (415) 436-9683 [email protected] 5 [email protected] 6 Collette L. Adkins Giese (MN Bar No. 035059X)* Center for Biological Diversity 8640 Coral Sea Street Northeast 7 Minneapolis, MN 55449-5600 Tel: (651) 955-3821 8 Fax: (415) 436-9683 [email protected] 9 Michael W. Graf (CA Bar No. 136172) 10 Law Offices 227 Behrens Street 11 El Cerrito, CA 94530 Tel: (510) 525-7222 12 Fax: (510) 525-1208 [email protected] 13 Attorneys for Plaintiffs Center for Biological Diversity and 14 Pesticide Action Network North America *Seeking admission pro hac vice 15 16 IN THE UNITED STATES DISTRICT COURT 17 FOR THE NORTHERN DISTRICT OF CALIFORNIA 18 SAN FRANCISCO DIVISION 19 20 CENTER FOR BIOLOGICAL ) 21 DIVERSITY, a non-profit organization; and ) Case No.__________________ PESTICIDE ACTION NETWORK ) 22 NORTH AMERICA, a non-profit ) organization; ) 23 ) Plaintiffs, ) COMPLAINT FOR DECLARATORY 24 ) AND INJUNCTIVE RELIEF v. ) 25 ) ENVIRONMENTAL PROTECTION ) 26 AGENCY; and LISA JACKSON, ) Administrator, U.S. EPA; ) 27 ) Defendants. ) 28 _____________________________________ ) Complaint for Declaratory and Injunctive Relief 1 1 INTRODUCTION 2 1. This action challenges the failure of Defendants Environmental Protection Agency and 3 Lisa Jackson, Environmental Protection Agency Administrator, (collectively “EPA”) to consult with the 4 United States Fish and Wildlife Service (“FWS”) and National Marine Fisheries Service (“NMFS”) 5 (collectively “Service”) pursuant to Section 7(a)(2) of the Endangered Species Act (“ESA”), 16 U.S.C. -

Crayfish News Volume 32 Issue 1-2: Page 1
June 2010 Volume 32, Issue 1-2 ISSN: 1023-8174 (print), 2150-9239 (online) The Official Newsletter of the International Association of Astacology Inside this issue: Cover Story 1 Searching for Crayfish in the President’s Corner 2 River Bug, Ukraine Info About IAA18 3 Future Directions 4 Symposium Info Short Articles 6 Male Form 6 Alternation in Spinycheek Crayfish, Orconectes limosus, at Cessy (East-central France): The Discovery of Anomalous Form Males IAA Related News 10 News Items From 11 Around the World Meeting 13 Announcements Literature of 16 Interest to Astacologists Figure 1. Astacus leptodactylus from the River Bug, Ukraine. comparison with other species, information n official opportunity for the author on A. pachypus is very limited (Souty-Grosset A to travel to the Ukraine was the 2nd et al., 2006). Since no specimens were meeting of the signatories to the available to be photographed for the “Memorandum of Understanding (MoU) on identification guide of crayfish species in the Conservation and Management of the Europe (Pöckl et al., 2006), only a sketch was middle European population of the Great presented. Bustard (Otis tarda)” under the “Convention Feodosia, located on the Crimean of Migratory Species of Wild Animals” (CMS th Peninsula, is not easily reached by airplane, or Bonn Convention) from November 8-12 with the nearest airport being in Simferopol. 2008. There are no direct flights to this region from The author has always dreamed of most European capitals, with connecting visiting the Ukraine in order to collect flights running through Kiev, Moscow or specimens of the thick-clawed crayfish, Istanbul. -

Checklist of the Crayfish and Freshwater Shrimp (Decapoda) of Indiana
2001. Proceedings of the Indiana Academy of Science 110:104-110 CHECKLIST OF THE CRAYFISH AND FRESHWATER SHRIMP (DECAPODA) OF INDIANA Thomas P. Simon: U.S. Fish and Wildlife Service, 620 South Walker Street, Bloomington, Indiana 47401 ABSTRACT. Crayfish and freshwater shrimp are members of the order Decapoda. All crayfish in In- diana are members of the family Cambaridae, while the freshwater shrimp belong to Palaemonidae. Two genera of freshwater shrimps, each represented by a single species, occur in Indiana. Palaemonetes ka- diakensis and Macrobrachium ohione are lowland forms. Macrobrachium ohione occurs in the Ohio River drainage, while P. kadiakensis occurs statewide in wetlands and lowland areas including inland lakes. Currently, 21 crayfish taxa, including an undescribed form of Cambarus diogenes, are found in Indiana. Another two species are considered hypothetical in occurrence. Conservation status is recommended for the Ohio shrimp Macrobrachium ohione, Indiana crayfish Orconectes indianensis, and both forms of the cave crayfish Orconectes biennis inennis and O. i. testii. Keywords: Cambaridae, Palaemonidae, conservation, ecology The crayfish and freshwater shrimp belong- fish is based on collections between 1990 and ing to the order Decapoda are among the larg- 2000. Collections were made at over 3000 lo- est of Indiana's aquatic invertebrates. Crayfish calities statewide, made in every county of the possess five pair of periopods, the first is mod- state, but most heavily concentrated in south- ified into a large chela and dactyl (Pennak ern Indiana, where the greatest diversity of 1978; Hobbs 1989). The North American species occurs. families, crayfish belong to two Astacidae and The current list of species is intended to Cambaridae with all members east of the Mis- provide a record of the extant and those ex- sissippi River belong to the family Cambari- tirpated from the fauna of Indiana over the last dae (Hobbs 1974a). -

First Report of Golden Crayfish Faxonius Luteus (Creaser, 1933) in South Dakota
BioInvasions Records (2021) Volume 10, Issue 1: 149–157 CORRECTED PROOF Rapid Communication First report of golden crayfish Faxonius luteus (Creaser, 1933) in South Dakota Gene Galinat1,*, Mael Glon2 and Brian Dickerson3 1South Dakota Department of Game, Fish and Parks, Rapid City, South Dakota, USA 2The Ohio State University Museum of Biological Diversity, Columbus, Ohio, USA 3U.S. Department of Agriculture Forest Service, Rocky Mountain Research Station, Rapid City, SD, 57702, USA Author e-mails: [email protected] (GG), [email protected] (MG), [email protected] (BD) *Corresponding author Citation: Galinat G, Glon M, Dickerson B (2021) First report of golden crayfish Abstract Faxonius luteus (Creaser, 1933) in South Dakota. BioInvasions Records 10(1): 149– The golden crayfish, Faxonius luteus, was identified for the first time in the Black 157, https://doi.org/10.3391/bir.2021.10.1.16 Hills of South Dakota. We collected specimens from three reservoirs and one stream in two adjacent watersheds. The species appears to be established with varying Received: 7 February 2020 sizes and Form I and Form II males being observed. Records show the home range Accepted: 20 August 2020 of F. luteus to be over 600 km east of the Black Hills. The lack of historic information Published: 1 December 2020 on aquatic fauna in the area complicates determining what effects F. luteus may Handling editor: David Hudson have on native and other non-native fauna in the area. Thematic editor: Karolina Bącela- Spychalska Key words: bait, baitfish, Black Hills, non-native Copyright: © Galinat et al. -
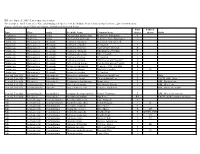
Threatened and Endangered Species List
Effective April 15, 2009 - List is subject to revision For a complete list of Tennessee's Rare and Endangered Species, visit the Natural Areas website at http://tennessee.gov/environment/na/ Aquatic and Semi-aquatic Plants and Aquatic Animals with Protected Status State Federal Type Class Order Scientific Name Common Name Status Status Habit Amphibian Amphibia Anura Gyrinophilus gulolineatus Berry Cave Salamander T Amphibian Amphibia Anura Gyrinophilus palleucus Tennessee Cave Salamander T Crustacean Malacostraca Decapoda Cambarus bouchardi Big South Fork Crayfish E Crustacean Malacostraca Decapoda Cambarus cymatilis A Crayfish E Crustacean Malacostraca Decapoda Cambarus deweesae Valley Flame Crayfish E Crustacean Malacostraca Decapoda Cambarus extraneus Chickamauga Crayfish T Crustacean Malacostraca Decapoda Cambarus obeyensis Obey Crayfish T Crustacean Malacostraca Decapoda Cambarus pristinus A Crayfish E Crustacean Malacostraca Decapoda Cambarus williami "Brawley's Fork Crayfish" E Crustacean Malacostraca Decapoda Fallicambarus hortoni Hatchie Burrowing Crayfish E Crustacean Malocostraca Decapoda Orconectes incomptus Tennessee Cave Crayfish E Crustacean Malocostraca Decapoda Orconectes shoupi Nashville Crayfish E LE Crustacean Malocostraca Decapoda Orconectes wrighti A Crayfish E Fern and Fern Ally Filicopsida Polypodiales Dryopteris carthusiana Spinulose Shield Fern T Bogs Fern and Fern Ally Filicopsida Polypodiales Dryopteris cristata Crested Shield-Fern T FACW, OBL, Bogs Fern and Fern Ally Filicopsida Polypodiales Trichomanes boschianum -

Rusty Crayfish (Orconectes Rusticus) Threatens the State of Michigan’S Waterways
State of Michigan’s Status and Strategy for Rusty Crayfish Management Scope The invasive rusty crayfish (Orconectes rusticus) threatens the State of Michigan’s waterways. The goals of this document are to: • Summarize current level of understanding on the biology and ecology of the rusty crayfish. • Summarize current management options for the rusty crayfish in Michigan. • Identify possible future directions of rusty crayfish management in Michigan. Biology and Ecology I. Identification Amy Benson - U.S. Geological Survey The freshwater crustacean known as the rusty crayfish can be difficult to identify and can be confused for other common crayfish species found in the Great Lakes Region. One distinguishing characteristic is the rusty crayfish’s claws, which are larger, more robust claws when compared to other crayfish, such as the papershell (O. immunis) and the northern crayfish (O. virilis) (Gunderson 1998). Furthermore, the rusty crayfish has smooth, grayish-green to reddish-brown claws; this is unlike the northern crayfish, which has blue colored claws with white bumps (Gunderson 1998). The dark, rusty spots on each side of the rusty crayfish’s carapace are a distinguishing characteristic, even though these spots are absent or not as distinct on individuals from some waters (Gunderson 1998). Rusty crayfish also have a rust-colored band down the center of the back side of the abdomen, black bands at the tips of their claws, and a gap in their claws when closed (Wetzel et al. 2004). While they share similar claws, the northern clearwater crayfish (O. propinquus) has a dark brown/black patch on the top of the tail section and lack the rusty crayfish’s side carapace spots (Gunderson 1998). -
![Kyfishid[1].Pdf](https://docslib.b-cdn.net/cover/2624/kyfishid-1-pdf-1462624.webp)
Kyfishid[1].Pdf
Kentucky Fishes Kentucky Department of Fish and Wildlife Resources Kentucky Fish & Wildlife’s Mission To conserve, protect and enhance Kentucky’s fish and wildlife resources and provide outstanding opportunities for hunting, fishing, trapping, boating, shooting sports, wildlife viewing, and related activities. Federal Aid Project funded by your purchase of fishing equipment and motor boat fuels Kentucky Department of Fish & Wildlife Resources #1 Sportsman’s Lane, Frankfort, KY 40601 1-800-858-1549 • fw.ky.gov Kentucky Fish & Wildlife’s Mission Kentucky Fishes by Matthew R. Thomas Fisheries Program Coordinator 2011 (Third edition, 2021) Kentucky Department of Fish & Wildlife Resources Division of Fisheries Cover paintings by Rick Hill • Publication design by Adrienne Yancy Preface entucky is home to a total of 245 native fish species with an additional 24 that have been introduced either intentionally (i.e., for sport) or accidentally. Within Kthe United States, Kentucky’s native freshwater fish diversity is exceeded only by Alabama and Tennessee. This high diversity of native fishes corresponds to an abun- dance of water bodies and wide variety of aquatic habitats across the state – from swift upland streams to large sluggish rivers, oxbow lakes, and wetlands. Approximately 25 species are most frequently caught by anglers either for sport or food. Many of these species occur in streams and rivers statewide, while several are routinely stocked in public and private water bodies across the state, especially ponds and reservoirs. The largest proportion of Kentucky’s fish fauna (80%) includes darters, minnows, suckers, madtoms, smaller sunfishes, and other groups (e.g., lam- preys) that are rarely seen by most people. -

Status of Native and Invasive Crayfish in Ten National Park Service Properties in Pennsylvania
National Park Service U.S. Department of the Interior Northeast Region Philadelphia, Pennsylvania Status of Native and Invasive Crayfish in Ten National Park Service Properties in Pennsylvania Technical Report NPS/NER/NRTR—2007/085 ON THE COVER Top left - Rusty crayfish (Orconectes rusticus); Top Right – A member of the Cambarus acuminatus species complex [Cambarus (Puncticambarus) sp.]; Bottom left - Marsh Creek, Eisenhower National Historic Site; Bottom right - Baptism Creek, Hopewell Furnace National Historic Site. Photographs by: David A. Lieb and Paula Mooney. Status of Native and Invasive Crayfish in Ten National Park Service Properties in Pennsylvania Technical Report NPS/NER/NRTR—2007/085 David A. Lieb1, Robert F. Carline2, and Hannah M. Ingram2 1Intercollege Graduate Degree Program in Ecology The Pennsylvania State University 435 Forest Resources Building University Park, Pennsylvania 16802 ([email protected]) 2Pennsylvania Cooperative Fish and Wildlife Research Unit U.S.G.S. Biological Resources Division The Pennsylvania State University 402 Forest Resources Building University Park, Pennsylvania 16802 April 2007 U.S. Department of the Interior National Park Service Northeast Region Philadelphia, Pennsylvania The Northeast Region of the National Park Service (NPS) comprises national parks and related areas in 13 New England and Mid-Atlantic states. The diversity of parks and their resources are reflected in their designations as national parks, seashores, historic sites, recreation areas, military parks, memorials, and rivers and trails. Biological, physical, and social science research results, natural resource inventory and monitoring data, scientific literature reviews, bibliographies, and proceedings of technical workshops and conferences related to these park units are disseminated through the NPS/NER Technical Report (NRTR) and Natural Resources Report (NRR) series. -

Biological Supply and Freshwater Invasive Species: Crayfish in The
Biological Supply and Freshwater Invasive Species: A Crayfish Case Study from the Pacific Northwest Eric R. Larson [email protected] Daniel P. Haerther Center for Conservation and Research, John G. Shedd Aquarium, Chicago, IL Environmental Change Initiative, University of Notre Dame Freshwater Invasive Species Canal building wisc sea grant epa.gov Shipping dnr.wi.gov Bait bucket Biological supply? ? Biological Supply and Freshwater Invasive Species Biological Supply and Freshwater Invasive Species Sam Chan [email protected] Pat Charlebois [email protected] Illinois-Indiana Sea Grant Biological Supply and Freshwater Invasive Species Invasive crayfish in the Pacific Northwest - Are organisms being introduced by biological supply? - What is the potential extent of the problem? - What are some solutions (and their complications)? Native species pilot program? 2001 Washington Department of Fish and Wildlife biologist (Karl Mueller) finds red swamp crayfish Procambarus clarkii in urban lake near Seattle (Pine Lake) Similar to later P. clarkii invasions in Wisconsin, Illinois 2007 I started my PhD to investigate distributions, impacts of invasive crayfish in Seattle area with Julian D. Olden INVASIVE NATIVE Procambarus clarkii Pacifastacus leniusculus Where11 established? lakes Where53 lakespresent? 4vulnerable? lakes, 3 species vulnerable? Orconectes virilis; Orconectes sanbornii; Procambarus acutus 100 lakes surveyed 2007-2009 trap x 5 LAKE EXAMPLE trap x 5 trap x 5 snorkel trap x 5 access 2009 Orconectes rusticus discovered west of Continental Divide for first time John Day River of Oregon Where are all of these invasive crayfish coming from? rumor that lots of schools in How do crayfish Seattle area were using live crayfish get introduced? I visited a school near U Washington campus… … that had three non- native species on site Procambarus clarkii banned in WA State no bait shops sold crayfish Orconectes virilis “We really like these (O. -

Etheostoma Maydeni) Species Status Assessment
Redlips Darter (Etheostoma maydeni) Species Status Assessment Version 1.1 Photo courtesy of Dr. David Neely, Tennessee Aquarium Conservation Institute (East Fork Obey River, Fentress County, Tennessee, December 2017) U.S Fish and Wildlife Service Region 4 Atlanta, Georgia May 2018 Redlips Darter SSA This document was prepared by Dr. Michael A. Floyd, U.S. Fish and Wildlife Service, Kentucky Ecological Services Field Office, Frankfort, Kentucky. The U.S. Fish and Wildlife Service greatly appreciates the assistance of Tom Barbour (Kentucky Division of Mine Permits), Darrell Bernd (Tennessee Wildlife Resources Agency (TWRA)), Davy Black (Eastern Kentucky University), Stephanie Brandt (Kentucky Department of Fish and Wildlife Resources (KDFWR)), Bart Carter (TWRA), Stephanie Chance (USFWS – Tennessee Ecological Services Field Office (TFO)), Brian Evans (USFWS – Atlanta), Mike Compton (Kentucky State Nature Preserves Commission (KSNPC)), Dr. Bernie Kuhajda (Tennessee Aquarium Conservation Institute (TNACI), Pam Martin (U.S. Forest Service - Daniel Boone National Forest (DBNF)), Matt Moran (U.S. Office of Surface Mining Reclamation and Enforcement), Dr. Dave Neely (TNACI), Dr. Steve Powers (Roanoke College), Pat Rakes (Conservation Fisheries, Inc. (CFI)), Rebecca Schapansky (National Park Service – Big South Fork National River and Recreation Area), J.R. Shute (CFI), Jeff Simmons (Tennessee Valley Authority (TVA)), Justin Spaulding (TWRA), Kurt Snider (USFWS – TFO), Dr. Matthew Thomas (KDFWR), Keith Wethington (KDFWR), David Withers (Tennessee Department of Environment and Conservation (TDEC)), and Brian Zimmerman (The Ohio State University), who provided helpful information and/or review of the draft document. Suggested reference: U.S. Fish and Wildlife Service. 2018. Redlips Darter (Etheostoma maydeni) Species Status Assessment, Version 1.1. May 2018.