COMMISSION DECISION of 24 February 2006 on Introducing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Publication of an Application for Approval of Amendments, Which Are
20.1.2020 EN Offi cial Jour nal of the European Union C 18/39 Publication of an application for approval of amendments, which are not minor, to a product specification pursuant to Article 50(2)(a) of Regulation (EU) No 1151/2012 of the European Parliament and of the Council on quality schemes for agricultural products and foodstuffs (2020/C 18/09) This publication confers the right to oppose the amendment application pursuant to Article 51 of Regulation (EU) No 1151/2012 of the European Parliament and of the Council (1) within three months from the date of this publication. APPLICATION FOR APPROVAL OF NON-MINOR AMENDMENTS TO THE PRODUCT SPECIFICATION FOR A PROTECTED DESIGNATION OF ORIGIN OR PROTECTED GEOGRAPHICAL INDICATION Application for approval of amendments in accordance with the first subparagraph of Article 53(2) of Regulation (EU) No 1151/2012 ‘JAMBON DE BAYONNE’ EU No: PGI-FR-00031-AM01 — 11.9.2018 PDO ( ) PGI (X) 1. Applicant group and legitimate interest Consortium du Jambon de Bayonne Route de Samadet 64 410 Arzacq FRANCE Tel. +33 559044935 Fax +33 559044939 Email: [email protected] Membership: Producers/processors 2. Member State or third country France 3. Heading in the product specification affected by the amendment(s) Name of product Description of product Geographical area Proof of origin Method of production Link Labelling Other: data update, inspection bodies, national requirements, annexes 4. Type of amendment(s) Amendments to the product specification of a registered PDO or PGI not to be qualified as minor within the meaning of the third subparagraph of Article 53(2) of Regulation (EU) No 1151/2012 Amendments to the product specification of a registered PDO or PGI for which a Single Document (or equivalent) has not been published and which cannot be qualified as minor within the meaning of the third subparagraph of Article 53(2) of Regulation (EU) No 1151/2012 (1) OJ L 343, 14.12.2012, p. -

Note Aubagnan
direction Service de Bureau départementale l’Environnement, Prévention des de l’Equipement des Risques Risques, des landes et de la Sécurité Aménagement Durable et Défense Dossier d’information sur le risque Inondation sur la commune d’ AUBAGNAN août 2008 Préface Etape finale de la démarche de l’Etat et maillon clé du droit à l’information des citoyens, ce dossier présente le risque inondation qui menace votre commune.... Le document a été élaboré et validé grâce aux données recueillies et aux connaissances détenues aujourd’hui par les services de l’Etat. Malgré ses limites, il a cependant le mérite de décrire et figurer le mieux possible le phénomène inondation. Ainsi, je souhaite que ce Dossier d’Information serve de base à une information la plus large possible des responsables et citoyens concernés. Le Préfet A) GENERALITES I) QU’EST-CE QU’UNE INONDATION ? Une inondation est une submersion, rapide ou lente, d’une zone habituellement hors d’eau. Le risque inondation est la conséquence de deux composantes : l’eau qui peut sortir de son lit habituel d’écoulement ou apparaître et l’homme qui s’installe dans la zone inondable pour y implanter toutes sortes de constructions, d’équipements et d’activités. II) COMMENT SE MANIFESTE –T-ELLE ? On distingue trois types d’inondations - La montée lente des eaux en région de plaine par débordement d’un cours d’eau ou remontée de la nappe phréatique . - La formation rapide de crues torrentielles consécutives à des averses violentes. - Le ruissellement pluvial renforcé par l’imperméabilisation des sols et les pratiques culturales limitant l’infiltration des précipitations. -

40990 Angoumé 40150 Angresse 40320 Arboucave 40110
40990 Angoumé 40150 Angresse 40320 Arboucave 40110 Arengosse 40700 Argelos 40430 Argelouse 40110 Arjuzanx 40330 Arsague 40190 Arthez-d'Armagnac 40120 Arue 40700 Aubagnan 40500 Audignon 40400 Audon 40320 Bahus-Soubiran 40380 Baigts 40700 Bassercles 40360 Bastennes 40320 Bats 40400 Bégaar 40410 Belhade 40120 Bélis 40300 Bélus 40180 Bénesse-lès-Dax 40230 Bénesse-Maremne 40250 Bergouey 40240 Betbezer-d'Armagnac 40370 Beylongue 40700 Beyries 40390 Biarrotte 40170 Bias 40390 Biaudos 40600 Biscarrosse 40330 Bonnegarde 40370 Boos 40270 Bordères-et-Lamensans 40190 Bourdalat 40330 Brassempouy 40320 Buanes 40120 Cachen 40300 Cagnotte 40430 Callen 40090 Campagne 40090 Canenx-et-Réaut 40400 Carcarès-Sainte-Croix 40400 Carcen-Ponson 40380 Cassen 40330 Castel-Sarrazin 40360 Castelnau-Chalosse 40320 Castelnau-Tursan 40700 Castelner 40500 Cauna 40300 Cauneille 40250 Caupenne 40700 Cazalis 40090 Cère 40320 Classun 40320 Clèdes 40180 Clermont 40210 Commensacq 40240 Créon-d'Armagnac 40360 Donzacq 40500 Dumes 40210 Escource 40290 Estibeaux 40240 Estigarde 40320 Eugénie-les-Bains 40500 Eyres-Moncube 40500 Fargues 40190 Frêche 40350 Gaas 40090 Gaillères 40380 Gamarde-les-Bains 40420 Garein 40180 Garrey 40110 Garrosse 40160 Gastes 40330 Gaujacq 40320 Geaune 40090 Geloux 40380 Gibret 40180 Goos 40990 Gourbera 40465 Gousse 40400 Gouts 40290 Habas 40700 Hagetmau 40300 Hastingues 40250 Hauriet 40180 Heugas 40190 Hontanx 40700 Horsarrieu 40230 Josse 40700 Labastide-Chalosse 40300 Labatut 40530 Labenne 40210 Labouheyre 40320 Lacajunte 40700 Lacrabe 40090 Laglorieuse -
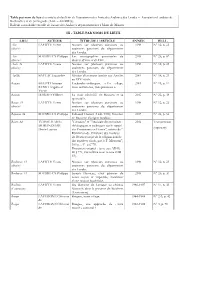
Aal Table Nom De Lieu 2018
Table par nom de lieu des articles du bulletin de l'association des Amis des Archives des Landes – Association Landaise de Recherches et de Sauvegarde (AAL – ALDRES). Bulletin consultable en salle de lecture des Archives départementales à Mont-de-Marsan. III - TABLE PAR NOMS DE LIEUX LIEU AUTEUR TITRE DE L’ARTICLE ANNÉE BULL. Aire LAFITTE Yvett e Notices sur plusieurs paroisses ou 1998 N° 12, p. 23 (diocèse) anciennes paroisses du département des Landes. Aire SOUSSIEUX Philippe Les monographies paroissiales du 2018 N° 26, p. 67 (diocèse) diocèse d’Aire et de Dax. Auch, 32 LAFITTE Yvette Notices sur plusieurs paroisses ou 1998 N° 12, p. 23 (diocèse) anciennes paroisses du département des Landes. Antilles BAYLAC Jacqueline Mission d’un marin landais aux Antilles 2000 N° 14, p. 23 au XIX e siècle. Bargues BELOT Christian Lucbardez -et-Bargues. « Un village, 2001 N° 15, p. 11 RUSSO Angello et deux communes, trois paroisses ». Yvette Bascons DARDEY Gilbert La mater admirabilis de Bascons et sa 2012 N° 22, p. 39 quenouille. Bazas, 33 LAFITTE Yvette Notices sur plusieurs paroisses ou 1998 N° 12, p. 23 (diocèse) anciennes paroisses du département des Landes. Bayonne , 64 SOU SSIEUX Philippe Édouard Ducéré (1849 -1910) historien 2015 N° 24, p . 84 de Bayonne d’origine landaise. Béarn , 64 TURMEAU de la "Critiques" et "Apologie des principes 2014 Transcri ption MORANDIERE théologiques et politiques sur le rappel (tapuscrit) Denis-Laurian des Protestants en France", suivies de " RéFutation de l'Histoire des troubles du Béarn au sujet de la religion dans le dix-septième siècle, par le P. -

Communes Actionnaires Mise À Jour Janvier 2017
Communes actionnaires Mise à jour janvier 2017 Cugand Noirmoutier en l'Ile La Bernardière Bouin La Bruffière L'Epine St Hilaire de Loulay Tiffauges St Philbert de Bouaine La Guérinière Treize Septiers St Aubin des Ormeaux Barbâtre Montaigu St Martin des Tilleuls Mortagne sur Sèvre Boufféré La Guyonnière Bois de Cené Les Landes Genusson La Verrie St Gervais St Laurent sur Sèvre Beauvoir sur Mer La Boissière de Montaigu Châteauneuf Rocheservière St Georges La Gaubretière de Montaigu St Malo du Bois Mallièvre Treize Vents La Garnache L'Herbergement Bazoges en Paillers St Urbain Chambretaud Montréverd La Barre de Monts Les Brouzils Chavagnes en Paillers Beaurepaire Les Epesses Sallertaine Froidfond Falleron Grand'Landes Les Lucs sur Boulogne La Rabatelière Les Herbiers Notre Dame de Monts La Copechagnière Challans St Fulgent St Mars la Réorthe St Etienne du Bois St Denis la Chevasse Mesnard la Barotière Le Perrier St Christophe du Ligneron Chauché Soullans St André St Paul en Pareds Sèvremont St Paul Mont Penit Beaufou Goule d'Oie Vendrennes St Jean de Monts Palluau La Chapelle Palluau Ste Florence Mouchamps Le Boupère Bellevigny Boulogne St Mesmin Commequiers L'Oie Maché Essarts- Rochetrejoux Pouzauges Notre Dame de Riez en-Bocage Le Poiré sur Vie Apremont Dompierre sur Yon St Hilaire de Riez La Merlatière St Prouant Montournais L'Ile d'Yeu St Maixent sur Vie Aizenay St Maixent sur Vie St Vincent Sterlanges La Meilleraie Tillay La Genétouze Le Fenouiller St Martin des Noyers St Germain de Prinçay Monsireigne St Révérend Coëx Menomblet Mouilleron -

Landes Randonnée® (Itinéraires Pédestres, VTT Et Équestres)
Les types de balisage ® Landes Randonnée (itinéraires pédestres, VTT et équestres) Les circuits de randonnée sont tous balisés sur le Offices de Tourisme terrain avec un code différent selon les pratiques Promenades de randonnée : à pied, à vélo ou à cheval. Aire-sur-l’Adour - 40800 et randonnées Tél : 05 58 71 64 70 - tourisme-aire-eugenie.fr Type de sentier Amou - 40330 dans les Landes Tél : 05 58 89 02 25 - amoutourisme.com Site Nature 40 Bonne Biscarrosse - 40600 direction Tél : 05 58 78 20 96 - biscarrosse.com À PIED, À CHEVAL OU À VÉLO : ou rappel À CHACUN SA RANDO ! Capbreton - 40130 Tél : 05 58 72 12 11 - capbreton-tourisme.com Tournez à Edition gauche Castets - 40260 2019 Tél : 05 58 89 44 79 - cotelandesnaturetourisme.com Tournez à Dax - 40100 droite Tél : 05 58 56 86 86 - dax-tourisme.com Eugénie-les-Bains - 40320 Mauvaise Tél : 05 58 51 13 16 - tourisme-aire-eugenie.fr direction Gabarret - 40310 Tél : 05 58 44 34 95 - tourisme-landesdarmagnac.fr Geaune - 40320 Tél : 05 58 44 42 00 - landes-chalosse.com Grenade-sur-l’Adour - 40270 Tél : 05 58 45 45 98 - cc-paysgrenadois.fr/tourisme Hagetmau - 40700 Tél : 05 58 79 38 26 - tourisme-hagetmau.com Hossegor - 40150 Tél : 05 58 41 79 00 - hossegor.fr Département Labastide-d’Armagnac - 40240 des Landes Tél : 05 58 44 67 56 - tourisme-landesdarmagnac.fr Les Actions Environnementales Labenne - 40530 Tél : 05 59 45 40 99 - landesatlantiquesud.com Département des Landes Comité départemental du tourisme Léon - 40550 Direction de l’Environnement équestre Tél : 05 58 48 76 03 - cotelandesnaturetourisme.com 23 rue Victor Hugo 40025 Mont-de-Marsan cedex 40090 BOUGUE Lit-et-Mixe - 40170 Tél : 05 58 06 09 70 Tél : 05 58 42 72 47 - cotelandesnaturetourisme.com Tél. -

Cahier Des Charges De L'appellation D'origine Contrôlée Tursan
Publié au BO le 1er décembre 2016 Cahier des charges de l’appellation d’origine contrôlée « TURSAN » homologué par le décret n° 2011- 1366 du 24 octobre 2011, modifié par le décret n°2013-1099 du 2 décembre 2013, modifié par arrêté du 10 novembre 2016 publié au JORF du 24 novembre 2016 CHAPITRE Ier I. - Nom de l'appellation Seuls peuvent prétendre à l'appellation d’origine contrôlée « Tursan », initialement reconnue en appellation d’origine vin délimité de qualité supérieure par arrêté du 11 juillet 1958, les vins répondant aux dispositions fixées ci-après. II. - Dénominations géographiques et mentions complémentaires Pas de disposition particulière. III. – Couleur et types de produit L’appellation d’origine contrôlée « Tursan » est réservée aux vins tranquilles blancs, rouges et rosés. IV. – Aires et zones dans lesquelles différentes opérations sont réalisées 1°- Aire géographique La récolte des raisins, la vinification et l’élaboration des vins sont assurées sur le territoire des communes suivantes : COMMUNES DONT LE TERRITOIRE, DANS SA TOTALITÉ, APPARTIENT À L’AIRE GÉOGRAPHIQUE - Dans le département du Gers : Ségos ; - Dans le département des Landes : Aubagnan, Bahus-Soubiran, Bats-Tursan, Buanes, Castelnau- Tursan, Classun, Clèdes, Coudures, Eugénie-les-Bains, Fargues, Geaune, Latrille, Lauret, Mauries, Miramont-Sensacq, , Montsoué, Payros-Cazautets, Pécorade, Puyol-Cazalet, Saint-Agnet, Saint- Loubouer, Sarraziet, Sarron, Serres-Gaston, Sorbets, Urgons, Vielle-Tursan. COMMUNES DONT LE TERRITOIRE APPARTIENT, POUR PARTIE, À L’AIRE GÉOGRAPHIQUE Dans le département des Landes : Aire-sur-l'Adour, Arboucave, Duhort-Bachen, Eyres-Moncube, Larrivière, Montgaillard, Pimbo, Renung. L’Institut national de l’origine et de la qualité dépose auprès des mairies des communes retenues en partie les documents graphiques établissant les limites de l’aire de production ainsi approuvées. -

CHALLENGE LANDAIS DES COURSES SUR ROUTE 2020 Manche N°3 - 107 F Et 277 M
CHALLENGE LANDAIS DES COURSES SUR ROUTE 2020 Manche N°3 - 107 F et 277 M - Clas. Nom Prénom Ville Année Sexe Points Epreuve Km Tot. 1 SALLABERRY MARGOT NICOLE LABATUT 1961 F 1375,00 3 34 2 MAZEROLLE EMILIE STADE MONTOIS F 1166,00 2 24 3 CANDAU FRANCOISE MONT DE MARSAN 1963 F 1164,00 3 34 4 JAVIERRE JEANINE GERDEREST 1958 F 1040,00 3 34 5 TOLOU CHRISTELLE GAAS 1975 F 1017,20 3 34 6 MAZAIN MADELEINE AC POUILLON F 1007,00 2 24 7 DUBECQ GENEVIEVE ESPOIR MUGRONNAIS RUNNING F 1004,00 2 24 8 BELLAMY NADEGE MUGRON 1976 F 999,00 3 34 9 LAMARQUE CECILE NARROSSE 1972 F 982,00 3 34 10 DAVERAT CELINE DOAZIT 1965 F 968,00 2 24 11 LAMOTHE FLORENCE F 966,00 2 24 12 FOURRIER CORINNE SAINT PAUL LES DAX 1964 F 943,00 3 34 13 DARGELOS SEVERINE MONT DE MARSAN 1971 F 920,00 2 20 14 BERTRAND MARIE PIERRE CAUPENNE D ARMAGNAC 1959 F 907,40 3 34 15 CIRON CAROLINE SAINT PIERRE DU MONT 1978 F 755,00 2 20 16 MARGUERITTE STEPHANIE MONT DE MARSAN 1979 F 735,00 2 20 17 DUMERGUES MARIE LAURE F 700,00 1 14 18 DELBARBA LYDIE LOS ASTIAUS F 672,00 1 14 19 DISCAZEAUX CORINNE BIARRITZ F 658,00 1 14 20 GUILLEMANE SOLENE ESPOIR MUGRONNAIS RUNNING F 644,00 1 14 21 GABRION SYLVIA TYROSSE F 630,00 1 14 22 PALASSIN MANCON ANGELIQUE F 616,00 1 14 23 AUDOUARD MARYLISE AVENIR ATURIN F 602,00 1 14 24 HERRERO CELINE JOG' IN MONT 2 F 588,00 1 14 25 DEHOORNE PRISCILLIA ESPOIR MUGRONNAIS RUNNING F 560,00 1 14 26 ISSARTEL BERNADETTE F 553,00 1 14 27 MAUDUIT ISABELLE BUANES 1970 F 543,40 2 24 28 CLEUET NADINE F 539,00 1 14 29 LAXAGUE SANDRINE F 532,00 1 14 30 MACHADO IMMA AMICALE LES 40 F -

La Liste Des RPI
La liste des RPI Extrait du SNUipp-FSU des Landes. https://40.snuipp.fr La liste des RPI - Infos Carrière - Mouvement - Mouvement 2010 - Date de mise en ligne : lundi 12 avril 2010 Description : Pour aider au mouvement. SNUipp-FSU des Landes. Copyright © SNUipp-FSU des Landes. Page 1/4 La liste des RPI DAX CENTRE LANDES AZUR, MESSANGES, MOLIETS LEVIGNACQ, UZA BEYLONGUE, CARCEN PONSON LESGOR, LALUQUE, TALLER ARENGOSSE,OUSSE SUZAN, ST YAGUEN,VILLENAVE RPC CARCARES, AUDON, GOUTS DAX SUD ADOUR ESTIBEAUX,MOUSCARDES,OSSAGES,TILH MIMBASTE, MISSON ORTHEVIELLE, PORT DE LANNE ORIST, PEY BELUS, ST ETIENNE D'ORTHE ST CRICQ DU GAVE, SORDE L'ABBAYE HASTINGUES, SAMES (64) CANDRESSE, NARROSSE ST VINCENT DE PAUL, TETHIEU HEUGAS, ST PANDELON MONT DE MARSAN HAUTE LANDE BOSTENS,POUYDESSEAUX, STE FOY,GAILLERES BELIS,BROCAS,CANENX,CERE, MAILLERES LUCBARDEZ, SAINT AVIT ARUE, CACHEN, LENCOUACQ BOURRIOT,LOSSE,RETJONS,VIELLE, SOUBIRAN,ST GOR GAREIN,LABRIT,LE SEN,VERT CREON, LABASTIDE LE FRECHE, ST JUSTIN LUXEY, SORE MONT DE MARSAN SUD ARMAGNAC ARTASSENX, BASCONS, BRETAGNE Copyright © SNUipp-FSU des Landes. Page 2/4 La liste des RPI BORDERES, CASTANDET, MAURRIN FARGUES, MONTGAILLARD BOURDALAT,HONTANX,ST GEIN PUJO, SAINT CRICQ VILLENEUVE BOUGUE, LAGLORIEUSE, MAZEROLLES MONT DE MARSAN SUD CHALOSSE AUBAGNAN, BATS, VIELLE TURSAN BRASSEMPOUY, SAINT CRICQ CHALOSSE HORSARRIEU, Ste COLOMBE, SERRES GASTON LACRABE,MANT,MORGANX,PEYRE,MONSEGUR, POUDENX ARSAGUE, BONNEGARDE, CASTEL SARRAZIN CASTAIGNOS, MOMUY, NASSIET BASTENNES,CASTELNAU,DONZACQ,GAUJACQ GAMARDE,GOOS,PRECHACQ GARREY, SORT EN CHALOSSE CASSEN,GOUSSE,LOUER,ONARD,ST GEOURS D'AURIBAT, ST JEAN DE LIER,VICQ D'AURIBAT LAUREDE, POYANNE CAUPENNE,LARBEY,MAYLIS,ST AUBIN HAURIET,MONTAUT,TOULOUZETTE AURICE, CAUNA, LAMOTHE, LE LEUY COUDURES,MONTSOUE,SARRAZIET AUDIGNON,BANOS,DUMES,EYRES MONCUBE MONT DE MARSAN TURSAN ASH PHILONDENX, URGONS PIMBO, SORBETS, MIRAMONT CAZERES, LE VIGNAU LARRIVIERE, RENUNG MIMIZAN PAYS DE BORN BIAS, MEZOS Copyright © SNUipp-FSU des Landes. -

Carte Touristique Des Landes 2019 2019 Landes Des Touristique Carte D | | | Biscarrosse E Tonneins Y Biscarrosse
le naturel LES LANDES Ouvert tous les jours jusqu’au 03/11/19 (Calendrier sur rhune.com) A B C D E F G H I J K L M N Léognan Aire-sur-l'Adour.................... J9 Maillères.............................H-i7 BASSIN LégendeSauveterre Amou..................................G10 Mano .................................... G3 1 -de-Guyenne Angoumé...............................D9 Mant ................................... H10 1 D’ARCACHON AngresseDuras ............................ C10 Marpaps .............................G10 Office de Tourisme Arboucave............................i10 Mauries ................................i10 Arengosse .............................F7 Maurrin...................................i8 Argelos...............................G10 Mauvezin-d'Armagnac...... J-K7 Musée de france Argelouse............................. G4 Maylis ................................... G9 Biganos Arjuzanx ................................F7 Mazerolles..............................i8 www.rhune.com Classé Patrimoine mondial de l'UNESCO Arsague.............................. F10 Mées......................................D9 La Teste- Artassenx...............................i8 Meilhan................................. G8 de-Buch Arthez-d'Armagnac .............. J8 Messanges ............................C8 Musée Arue........................................i6 Mézos ....................................D6 Cap Ferret Arx......................................... L6 Mimbaste............................ E10 CarteLe Barp touristique Miramon Aubagnan...................... -

Armagnac 2019 De Septembre À Décembre
Fêtez l’esprit de l’Armagnac 2019 De septembre à décembre Rdv au village Armagnac en Fête Rdv au chai Autour de l’alambic Rdv gastronomique L’automne gourmand En savoir plus… www.armagnac.fr L’Armagnac www.qualitelandes.com Rencontre avec tourisme-aquitaine.fr Savoir apprécier “Je rends l’homme joyeux Prenez le temps. Lentement vieilli au au-dessus de tout” ... fil des années, je suis unique, on ne me consomme pas mais on me Selon Vital Dufour, prieur déguste, tous les sens en éveil. d’Eauze en 1310, j’ai 40 vertus L’œil… observez ma teinte pour peu qu’un homme “timide et ma brillance, faites-moi [me] boit de temps en temps”. tourner dans votre verre. Je suis l’Armagnac, j’ai 700 ans Le nez… chauffez votre verre au creux de la main et laissez et beaucoup de choses monter dans vos narines à vous dire… De l’enfance mes premières effluves. Les papilles… humectez votre à la maturité langue de quelques gouttes et L’art de ma fabrication réside dans le bouche close appréciez. Lorsque je respect des traditions. Trois étapes sont suis bien vieilli, je donne, après le cruciales pour mon épanouissement : premier feu de l’alcool, une impression de suavité. La longueur en bouche La vinification : les raisins sont est plus liée à l’âge qu’à la qualité. pressés puis vinifiés de façon Le fond de verre… chauffez à traditionnelle et naturelle (le soufrage nouveau au creux des mains le “fond du vin et la chaptalisation sont interdits) de verre” vous sentirez tout mon bouquet : pruneau, vanille, noisette.. -

Itinerary Timetable
ITINERARY TIMETABLE KM STAGE 19 TIMETABLE To be run Run Caravan 45 kph 43 kph 41 kph DISTANCE FROM NEUTRALISED PYRÉNÉES-ATLANTIQUES (64) NEUTRALISED TO OFFICIAL START D281 MOURENX (D281-D9) START 10:20 12:20 12:20 12:20 D9 Quartier des Crêtes (OS-MARSILLON) 4.4km Quartier Bachard (ABIDOS) LAGOR CATEGORISED CLIMB 207 0 MOURENX RACE 10:30 12:30 12:30 12:30 START Km 12.1 - Côte de Bareille 202.1 4.9 MASLACQ (D9-VC-D275) 10:37 12:36 12:37 12:37 Climb of 1.9km at 5.1% 199.1 7.9 D275 ARGAGNON (near) (D275-D817) 10:41 12:40 12:41 12:41 198.1 8.9 D817 Gouze (MONT) (D817-D275) 10:43 12:42 12:42 12:43 RELAIS-ÉTAPE 194.9 12.1 D275 Côte de Bareille 10:48 12:46 12:47 12:48 193.1 13.9 ARTHEZ-DE-BÉARN (D275-D946-D731-D31-D946) 10:50 12:48 12:49 12:50 3, allée de Lattre de Tassigny 189.9 17.1 D946 N'haux 10:55 12:53 12:54 12:55 33410 Cadillac 188.9 18.1 Crossroad D946-D31 10:56 12:54 12:55 12:56 Km 168,4 185.8 21.2 D31 HAGETAUBIN (near) (D31-D945) 11:01 12:58 13:00 13:01 182.8 24.2 D945 LACADÉE 11:05 13:02 13:04 13:05 ROUTE FOR 180.2 26.8 SAULT-DE-NAVAILLES (D945-D101) 11:09 13:06 13:07 13:09 NON-RACE VEHICLES LANDES (40) 173.8 33.2 D933 S CASTAIGNOS-SOUSLENS (near) 11:18 13:14 13:16 13:18 Exit directly from the off-course 170.3 36.7 MOMUY 11:24 13:19 13:21 13:24 parking area (don’t follow the race 166.3 40.7 HAGETMAU (D933 S-VC-D933 S) 11:29 13:24 13:27 13:29 route).