Agriculture Is Very Important in Uganda As the Main Source Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

26TH – 28TH SEPTEMBER, 2016 MONDAY 26TH SEPTEMBER, 2016 7.00 – 9.00 Arrival and Registration A.M
PROGRAMME: 1st INTERNATIONAL SCIENTIFIC CONFERENCE ON BIO-WASTE RECYCLING IN UGANDA 26TH – 28TH SEPTEMBER, 2016 MONDAY 26TH SEPTEMBER, 2016 7.00 – 9.00 Arrival and Registration a.m. SESSION 1: OPENING CEREMONY: KATONGA HALL CHAIR MR. MATHIAS MULUMBA/VINCENT KISENYI 9.00 - 9.20 Anthems: Uganda and EAC am Opening Prayer Cultural presentation Sponsors messages 9.20 - 10.40 Welcome remarks: Conference Convenor, Deputy Vice Chancellor, Dr Frederick Kakembo am Remarks: Vice Chancellor, Prof Eriabu Lugujjo Keynote Address: Dr. Markus Francke, Promotion of Renewable Energy and Energy Efficiency Programme GIZ Official Opening Address: H.E. Yoweri Kaguta Museveni, President of the Republic of Uganda Group Photograph Opening of Exhibition 10.40 – Tea Break 11.00 a.m. SESSION 2: KATONGA HALL CHAIR: Eng. Christopher Ndawula 11.00 - Dr. Tom Okurut, National Environment Management Authority 11.20 a.m. 11.20 – Agriculture & Renewable Energy Research and Training Institute, Prof. Friedrich Rakow, Uganda Industrial 11.40 a.m. Research Institute 11.40 – Potential Resources Recovery Options from Waste Water Treatment: Case study from NWSC 12.00 noon Dr. Irene Mugabi, NWSC 12.00 – Building Inclusive Green Economies: Policy Drivers and Best Practices 12.20 p.m. Dr. Abubakar Moki, Cabinet Secretariat- Office of the President 12.20 – Presentation from Biogas Practitioner 12.40 p.m. 12.20 -1.00 Question and Answer p.m. 1.00 p.m. - Lunch Break 2.00 p.m. SESSION 3 SEZIBWA HALL: FOOD AND KATONGA HALL: ORANGE HALL: ZAMBEZI HALL: CROSS AGRIC. ENERGY WATER & CUTTING ISSUES ENVIRONMENT Chair: Dr. Kephas Nowakunda Chair: Dr. Albert Chair: Ms. -

UGANDA: PLANNING MAP (Details)
IMU, UNOCHA Uganda http://www.ugandaclusters.ug http://ochaonline.un.org UGANDA: PLANNING MAP (Details) SUDAN NARENGEPAK KARENGA KATHILE KIDEPO NP !( NGACINO !( LOPULINGI KATHILE AGORO AGU FR PABAR AGORO !( !( KAMION !( Apoka TULIA PAMUJO !( KAWALAKOL RANGELAND ! KEI FR DIBOLYEC !( KERWA !( RUDI LOKWAKARAMOE !( POTIKA !( !( PAWACH METU LELAPWOT LAWIYE West PAWOR KALAPATA MIDIGO NYAPEA FR LOKORI KAABONG Moyo KAPALATA LODIKO ELENDEREA PAJAKIRI (! KAPEDO Dodoth !( PAMERI LAMWO FR LOTIM MOYO TC LICWAR KAPEDO (! WANDI EBWEA VUURA !( CHAKULYA KEI ! !( !( !( !( PARACELE !( KAMACHARIKOL INGILE Moyo AYUU POBURA NARIAMAOI !( !( LOKUNG Madi RANGELAND LEFORI ALALI OKUTI LOYORO AYIPE ORAA PAWAJA Opei MADI NAPORE MORUKORI GWERE MOYO PAMOYI PARAPONO ! MOROTO Nimule OPEI PALAJA !( ALURU ! !( LOKERUI PAMODO MIGO PAKALABULE KULUBA YUMBE PANGIRA LOKOLIA !( !( PANYANGA ELEGU PADWAT PALUGA !( !( KARENGA !( KOCHI LAMA KAL LOKIAL KAABONG TEUSO Laropi !( !( LIMIDIA POBEL LOPEDO DUFILE !( !( PALOGA LOMERIS/KABONG KOBOKO MASALOA LAROPI ! OLEBE MOCHA KATUM LOSONGOLO AWOBA !( !( !( DUFILE !( ORABA LIRI PALABEK KITENY SANGAR MONODU LUDARA OMBACHI LAROPI ELEGU OKOL !( (! !( !( !( KAL AKURUMOU KOMURIA MOYO LAROPI OMI Lamwo !( KULUBA Koboko PODO LIRI KAL PALORINYA DUFILE (! PADIBE Kaabong LOBONGIA !( LUDARA !( !( PANYANGA !( !( NYOKE ABAKADYAK BUNGU !( OROM KAABONG! TC !( GIMERE LAROPI PADWAT EAST !( KERILA BIAFRA !( LONGIRA PENA MINIKI Aringa!( ROMOGI PALORINYA JIHWA !( LAMWO KULUYE KATATWO !( PIRE BAMURE ORINJI (! BARINGA PALABEK WANGTIT OKOL KINGABA !( LEGU MINIKI -

Conference Abstracts
2016 Conference Abstracts Ndejje University 1st International Scientific Conference on Bio-waste recycling in Uganda 26 – 28 September 2016 1. Automated Livestock Tracking and Management System: Makerere University (CEDAT), School of Engineering, Department of Electrical and Computer Engineering By J. Nayebare1, A. Niyonzima2, P. Bogere3 Successful farming has always required intense manual labour and acute management skills. Technological advancements of agricultural revolutions reduced the quantity of manual labour required but human direction is still necessary. A main component of these new strategies is livestock monitoring information. Animal tracking provides valuable information including recent location, movement and feeding patterns, and land usage. The collection and storage of this information as well as actions based upon the information are becoming more automated. In this research project, a system prototype for tracking and recording livestock was developed. The prototype requires attaching an animal with an RFID tag assigned a unique ID. Different read locations (e.g. barn, dip, field, and gate) assigned RFID readers that read from the RFID tag. A tag read by a reader determines the tag’s location and, therefore, that of the animal. A programmed microcontroller, with connected readers, processes data from the RFID tag. A Wi-Fi connected microcontroller forwards the data of a tag, location and timestamp to a mobile application and web application in real-time, thereby availing data from all the read stations. 1st International Scientific Conference on Bio-waste recycling in Uganda – 2016 Ndejje University 2. A smart Habitat for Honeybee: Makerere University (CEDAT) Department of Electrical and Computer Engineering, O.E.L. Buza1, A. -

ORTHODOX-CHURCH-UGANDA.Pdf
PUBLICATION: ΕΚΔΟΣΙΣ: HOLY METROPOLIS OF KAMPALA AND ALL UGANDA ΙΕΡΑ ΜΗΤΡΟΠΟΛΙΣ ΚΑΜΠΑΛΑΣ ΚΑΙ ΠΆΣΗΣ ΟΥΓΚΑΝΤΑΣ P.OBOX 3970 KAMPALA Tel: +256 414 542461 P,O,BOX 3970 KAMPALA Fax: “ “ “ TEL: +256 414 542461 E-mail: [email protected] FAX: +256 414 542461 E-mail : [email protected] PHOTOGRAPHIC ARCHIVE, INFORMATION MATERIAL ΦΩΤΟΓΡΑΦΙΚΟΝ ΑΡΧΕΙΟΝ / ΠΛΗΡΟΦΟΡΙΚΟΝ ΥΛΙΚΟΝ SOURCE: ΠΗΓΗ: Parishes, sub-parishes, priests, Laity Parish Nuclei, Holy Churches, Mysteries Ενορίες, Υπενορίες, Ιερείς, Λαικοί Ceremonies, Celibates, Commisions Ενοριακοί πυρήνες,Ιεροί Ναοί, Μυστήρια Τελετές, Μονάζοντες, Μονάζοντες Schools, Dormitories, Orphanages Επιτροπείες, Συμβούλια Learners, Teaching Personnel Helpers, Ground Pitches Σχολεία, Οικοτροφεία, Ορφανοτροφεία Μαθητευόμενοι. Διδακτικό Προσωπικό Patients,Doctors, Nurses, Health Centres Βοηθοί, Γήπεδα Unit of Roaming Doctors Groups of Mission Doctors Ασθενείς,Ιατροί, Νοσοκόμοι, Κέντρα Υγείας Περιφερόμενη Ιατρική Μονάδα Youth Union, Mothers Union/ladies Ιεραποστολικές Ιατρικές Ομάδες Non Governmental Organisation UOCCAP Publishing House, Social Centres Ένωση Νεολαίας, Ένωση Μητέρων/ Γυναικών Construction groups Μή Κυβερνητικός Οργανισμός UOCCAP Cultivations,Animal Husbandry, catle Εκδοτικός Οίκος, Κέντρα Εκδηλώσεων breeding Office property and Land Οικοδομικές Ομάδες Central Diaconates Καλλιέργειες, Κτηνοτροφίες EDITORIAL COMMITTEE: Γραφείο / Τμήμα Κτημάτων και Οικοπέδων Mr. Theodore KATO, (Theologian), Secretary General IMK-Uganda Κεντρικά Διακονήματα¨ Mr. Richard BAYEGO (Teacher), Asst. Secretary, -

Rcdf Projects in Luwero District, Uganda
Rural Communications Development Fund (RCDF) RCDF PROJECTS IN LUWERO DISTRICT, UGANDA MA P O F L UW E R O D IS T R IC T S H O W IN G S U B C O U N T IE S N Kam ira Butu ntu m ula Kiky us a Luw e ro TC Luwe ro Katik am u Zirobwe W ob ule nz i T C Bam una nika M ak ulubita N yim bw a Kalaga la Bom bo TC 10 0 10 20 Km s UCC Support through the RCDF Programme Uganda Communications Commission Plot 42 -44, Spring road, Bugolobi P.O. Box 7376 Kampala, Uganda Tel: + 256 414 339000/ 312 339000 Fax: + 256 414 348832 E-mail: [email protected] Website: www.ucc.co.ug 1 Table of Contents 1- Foreword……………………………………………………………….……….………..…..…....….…3 2- Background…………………………………….………………………..…………..….….……...……4 3- Introduction………………….……………………………………..…….…………….….…….……..4 4- Project profiles……………………………………………………………………….…..…….……...5 5- Stakeholders’ responsibilities………………………………………………….….…........…12 6- Contacts………………..…………………………………………….…………………..…….……….13 List of tables and maps 1- Table showing number of RCDF projects in Luwero district………..…….…….….5 2- Map of Uganda showing Luwero district………..………………….………..…...…….14 10- Map of Luwero district showing sub counties………..……………..……………….15 11- Table showing the population of Luwero district by sub counties…………..15 12- List of RCDF Projects in Luwero district…………………………………….……………16 Abbreviations/Acronyms UCC Uganda Communications Commission RCDF Rural Communications Development Fund USF Universal Service Fund MCT Multipurpose Community Tele-centre PPDA Public Procurement and Disposal Act of 2003 POP Internet Points of Presence ICT Information and Communications Technology UA Universal Access MoES Ministry of Education and Sports MoH Ministry of Health DHO District Health Officer CAO Chief Administrative Officer RDC Resident District Commissioner 2 1. -

Assessment Form
Local Government Performance Assessment Luwero District (Vote Code: 532) Assessment Scores Crosscutting Minimum Conditions 68% Education Minimum Conditions 60% Health Minimum Conditions 50% Water & Environment Minimum Conditions 65% Micro-scale Irrigation Minimum Conditions 70% Crosscutting Performance Measures 64% Educational Performance Measures 54% Health Performance Measures 30% Water & Environment Performance Measures 43% Micro-scale Irrigation Performance Measures 14% 532 Crosscutting Performance Luwero Measures 2020 District Summary of No. Definition of compliance Compliance justification Score requirements Local Government Service Delivery Results 1 4 Service Delivery • Evidence that infrastructure There was evidence that infrastructure projects Outcomes of DDEG projects implemented using implemented using DDEG funding were functional investments DDEG funding are functional and utilised as per the purpose as follows: and utilized as per the Maximum 4 points on purpose of the project(s): 1. Construction of 2 classroom block at Bombo Mixed this performance Primary UGX 58,762,993- Complete and functional; measure • If so: Score 4 or else 0 2. Completion of 3 classroom block and office at Luzeke COU primary school UGX 63,551,186 Complete and functional ; and 3. Construction of 5 stance pit latrine in 10 primary school - Kayindo P/S, Nambi Ummea Primary schoo, Nakabululu , Primary School, Gelyanda Primary school, Damascus Primary School, Kyentume COU Primary school , Lukomero COU Primary School , Nyimbwa CoU primary school , Nandeera Girls Primary School, Kikunhy- Kabungu Primary school, UGX 149,041,660 complete and functional . 2 Not applicable 0 Service Delivery a. If the average score in the Performance overall LLG performance assessment increased from Maximum 6 points on previous assessment : this performance measure o by more than 10%: Score 3 o 5-10% increase: Score 2 o Below 5 % Score 0 2 3 Service Delivery b. -

THE UGANDA GAZETTE [13Th J Anuary
The THE RH Ptrat.ir OK I'<1 AND A T IE RKPt'BI.IC OF UGANDA Registered at the Published General Post Office for transmission within by East Africa as a Newspaper Uganda Gazette A uthority Vol. CX No. 2 13th January, 2017 Price: Shs. 5,000 CONTEXTS P a g e General Notice No. 12 of 2017. The Marriage Act—Notice ... ... ... 9 THE ADVOCATES ACT, CAP. 267. The Advocates Act—Notices ... ... ... 9 The Companies Act—Notices................. ... 9-10 NOTICE OF APPLICATION FOR A CERTIFICATE The Electricity Act— Notices ... ... ... 10-11 OF ELIGIBILITY. The Trademarks Act—Registration of Applications 11-18 Advertisements ... ... ... ... 18-27 I t is h e r e b y n o t if ie d that an application has been presented to the Law Council by Okiring Mark who is SUPPLEMENTS Statutory Instruments stated to be a holder of a Bachelor of Laws Degree from Uganda Christian University, Mukono, having been No. 1—The Trade (Licensing) (Grading of Business Areas) Instrument, 2017. awarded on the 4th day of July, 2014 and a Diploma in No. 2—The Trade (Licensing) (Amendment of Schedule) Legal Practice awarded by the Law Development Centre Instrument, 2017. on the 29th day of April, 2016, for the issuance of a B ill Certificate of Eligibility for entry of his name on the Roll of Advocates for Uganda. No. 1—The Anti - Terrorism (Amendment) Bill, 2017. Kampala, MARGARET APINY, 11th January, 2017. Secretary, Law Council. General N otice No. 10 of 2017. THE MARRIAGE ACT [Cap. 251 Revised Edition, 2000] General Notice No. -

Experiences of Women War-Torture Survivors in Uganda: Implications for Health and Human Rights Helen Liebling-Kalifani
Journal of International Women's Studies Volume 8 | Issue 4 Article 1 May-2007 Experiences of Women War-Torture Survivors in Uganda: Implications for Health and Human Rights Helen Liebling-Kalifani Angela Marshall Ruth Ojiambo-Ochieng Nassozi Margaret Kakembo Follow this and additional works at: http://vc.bridgew.edu/jiws Part of the Women's Studies Commons Recommended Citation Liebling-Kalifani, Helen; Marshall, Angela; Ojiambo-Ochieng, Ruth; and Kakembo, Nassozi Margaret (2007). Experiences of Women War-Torture Survivors in Uganda: Implications for Health and Human Rights. Journal of International Women's Studies, 8(4), 1-17. Available at: http://vc.bridgew.edu/jiws/vol8/iss4/1 This item is available as part of Virtual Commons, the open-access institutional repository of Bridgewater State University, Bridgewater, Massachusetts. This journal and its contents may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden. ©2007 Journal of International Women’s Studies. Experiences of Women War-Torture Survivors in Uganda: Implications for Health and Human Rights By Helen Liebling-Kalifani,1 Angela Marshall,2 Ruth Ojiambo-Ochieng,3 and Nassozi Margaret Kakembo4 The effect of the aggressive rapes left me with constant chest, back and abdominal pain. I get some treatment but still, from time to time it starts all over again. It was terrible (Woman discussing the effects of civil war during a Kamuli Parish focus group). Amongst the issues treated as private matters that cannot be regulated by international norms, violence against women and women‟s health are particularly critical. -

Local Government Councils' Performance and Public Service
LOCAL GOVERNMENT COUNCILS’ PERFORMANCE AND PUBLIC SERVICE DELIVERY IN UGANDA Wakiso District Council Score-Card Report 2011/2012 Susan Namara - Wamanga Martin Kikambuse Ssali Peninah Kansiime ACODE Public Service Delivery and Accountability Report Series No.3, 2013 LOCAL GOVERNMENT COUNCILS’ PERFORMANCE AND PUBLIC SERVICE DELIVERY IN UGANDA Wakiso District Council Score-Card Report 2011/2012 Susan Namara - Wamanga Martin Kikambuse Ssali Peninah Kansiime ACODE Public Service Delivery and Accountability Report Series No.3, 2013 Published by ACODE P. O. Box 29836, Kampala Email: [email protected]; [email protected] Website: http://www.acode-u.org Citation: Namara-Wamanga, S., et.al., (2013). Local Government Councils’ Performance and Public Service Delivery in Uganda: Wakiso District Council Score-Card Report 2011/12. ACODE Public Service Delivery and Accountability Report Series No.3, 2013. Kampala. © ACODE 2013 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means electronic, mechanical, photocopying, recording or otherwise without the prior written permission of the publisher. ACODE policy work is supported by generous donations and grants from bilateral donors and charitable foundations. The reproduction or use of this publication for academic or charitable purposes or for purposes of informing public policy is excluded from this restriction. ISBN 978-9970-07-022-0 Wakiso District Council Score-Card Report 2011/12 Wakiso District Council Score-Card -
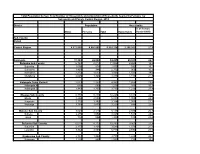
Population by Parish
Total Population by Sex, Total Number of Households and proportion of Households headed by Females by Subcounty and Parish, Central Region, 2014 District Population Households % of Female Males Females Total Households Headed HHS Sub-County Parish Central Region 4,672,658 4,856,580 9,529,238 2,298,942 27.5 Kalangala 31,349 22,944 54,293 20,041 22.7 Bujumba Sub County 6,743 4,813 11,556 4,453 19.3 Bujumba 1,096 874 1,970 592 19.1 Bunyama 1,428 944 2,372 962 16.2 Bwendero 2,214 1,627 3,841 1,586 19.0 Mulabana 2,005 1,368 3,373 1,313 21.9 Kalangala Town Council 2,623 2,357 4,980 1,604 29.4 Kalangala A 680 590 1,270 385 35.8 Kalangala B 1,943 1,767 3,710 1,219 27.4 Mugoye Sub County 6,777 5,447 12,224 3,811 23.9 Bbeta 3,246 2,585 5,831 1,909 24.9 Kagulube 1,772 1,392 3,164 1,003 23.3 Kayunga 1,759 1,470 3,229 899 22.6 Bubeke Sub County 3,023 2,110 5,133 2,036 26.7 Bubeke 2,275 1,554 3,829 1,518 28.0 Jaana 748 556 1,304 518 23.0 Bufumira Sub County 6,019 4,273 10,292 3,967 22.8 Bufumira 2,177 1,404 3,581 1,373 21.4 Lulamba 3,842 2,869 6,711 2,594 23.5 Kyamuswa Sub County 2,733 1,998 4,731 1,820 20.3 Buwanga 1,226 865 2,091 770 19.5 Buzingo 1,507 1,133 2,640 1,050 20.9 Maziga Sub County 3,431 1,946 5,377 2,350 20.8 Buggala 2,190 1,228 3,418 1,484 21.4 Butulume 1,241 718 1,959 866 19.9 Kampala District 712,762 794,318 1,507,080 414,406 30.3 Central Division 37,435 37,733 75,168 23,142 32.7 Bukesa 4,326 4,711 9,037 2,809 37.0 Civic Centre 224 151 375 161 14.9 Industrial Area 383 262 645 259 13.9 Kagugube 2,983 3,246 6,229 2,608 42.7 Kamwokya -

An Analysis of Uganda's Truth Commission
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by YorkSpace The Politics of Acknowledgement: An Analysis of Uganda’s Truth Commission Joanna R. Quinn1 Doctoral Candidate Department of Political Science McMaster University YCISS Working Paper Number 19 March 2003 The YCISS Working Paper Series is designed to stimulate feedback from other experts in the field. The series explores topical themes that reflect work being undertaken at the Centre. 1 I wish to thank Dr. Rhoda Howard-Hassmann of McMaster University for our on-going dialogue on this subject. I also gratefully acknowledge the support of Dr. William Coleman and the Institute on Globalization and the Human Condition of McMaster University, and of the International Development Research Centre in Ottawa, Canada. In the aftermath of a period of gross atrocity at the hands of the state, the restoration of the political and social fabric of a country is a pressing need. In the case of Uganda from the mid-1960s forward, this need was particularly real. Almost since the country had gained independence from Britain in 1962, a series of brutal governmental regimes had ransacked the country, and had viciously dealt with its inhabitants. Nearly thirty years of mind-numbing violence, perpetrated under the regimes of Idi Amin and Milton Obote, culminated in a broken society. Where once had stood a capable people, able to provide for themselves on every level, now was found a country whose economic, political, and social systems were seriously fractured. Under both Obote and Amin, as well as the transitional governments in place between and immediately following these regimes, democracy and the rule of law had been suspended. -

REPORT LG Consolidated
Local Government Quarterly Performance Report Vote: 532 Luwero District 2012/13 Quarter 2 Structure of Quarterly Performance Report Summary Quarterly Department Workplan Performance Cumulative Department Workplan Performance Location of Transfers to Lower Local Services and Capital Investments Submission checklist I hereby submit _________________________________________________________________________. This is in accordance with Paragraph 8 of the letter appointing me as an Accounting Officer for Vote:532 Luwero District for FY 2012/13. I confirm that the information provided in this report represents the actual performance achieved by the Local Government for the period under review. Name and Signature: Chief Administrative Officer, Luwero District Date: 6/7/2013 cc. The LCV Chairperson (District)/ The Mayor (Municipality) Page 1 Local Government Quarterly Performance Report Vote: 532 Luwero District 2012/13 Quarter 2 Summary: Overview of Revenues and Expenditures Overall Revenue Performance Cumulative Receipts Performance Approved Budget Cumulative % Receipts Budget UShs 000's Received 1. Locally Raised Revenues 317,627 104,635 33% 2a. Discretionary Government Transfers 2,805,648 1,286,050 46% 2b. Conditional Government Transfers 24,660,118 12,465,734 51% 2c. Other Government Transfers 1,328,176 223,101 17% 3. Local Development Grant 730,733 349,928 48% 4. Donor Funding 4,531,644 514,177 11% Total Revenues 34,373,945 14,943,626 43% Overall Expenditure Performance Cumulative Releases and Expenditure Perfromance Approved Budget Cumulative