Waveform Capnography • Pulse Oximetry • Colorimetric End-Tidal CO2 Monitoring
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Patient-Monitoring Systems
17 Patient-Monitoring Systems REED M. GARDNER AND M. MICHAEL SHABOT After reading this chapter,1 you should know the answers to these questions: ● What is patient monitoring, and why is it done? ● What are the primary applications of computerized patient-monitoring systems in the intensive-care unit? ● How do computer-based patient monitors aid health professionals in collecting, analyzing, and displaying data? ● What are the advantages of using microprocessors in bedside monitors? ● What are the important issues for collecting high-quality data either automatically or manually in the intensive-care unit? ● Why is integration of data from many sources in the hospital necessary if a computer is to assist in critical-care-management decisions? 17.1 What Is Patient Monitoring? Continuous measurement of patient parameters such as heart rate and rhythm, respira- tory rate, blood pressure, blood-oxygen saturation, and many other parameters have become a common feature of the care of critically ill patients. When accurate and imme- diate decision-making is crucial for effective patient care, electronic monitors frequently are used to collect and display physiological data. Increasingly, such data are collected using non-invasive sensors from less seriously ill patients in a hospital’s medical-surgical units, labor and delivery suites, nursing homes, or patients’ own homes to detect unex- pected life-threatening conditions or to record routine but required data efficiently. We usually think of a patient monitor as something that watches for—and warns against—serious or life-threatening events in patients, critically ill or otherwise. Patient monitoring can be rigorously defined as “repeated or continuous observations or meas- urements of the patient, his or her physiological function, and the function of life sup- port equipment, for the purpose of guiding management decisions, including when to make therapeutic interventions, and assessment of those interventions” (Hudson, 1985, p. -

Capnography 101 Oxygenation and Ventilation
It’s Time to Start Using it! Capnography 101 Oxygenation and Ventilation What is the difference? Oxygenation and Ventilation Ventilation O Oxygenation (capnography) 2 (oximetry) CO Cellular 2 Metabolism Capnographic Waveform • Capnograph detects only CO2 from ventilation • No CO2 present during inspiration – Baseline is normally zero CD AB E Baseline Capnogram Phase I Dead Space Ventilation • Beginning of exhalation • No CO2 present • Air from trachea, posterior pharynx, mouth and nose – No gas exchange occurs there – Called “dead space” Capnogram Phase I Baseline A B I Baseline Beginning of exhalation Capnogram Phase II Ascending Phase • CO2 from the alveoli begins to reach the upper airway and mix with the dead space air – Causes a rapid rise in the amount of CO2 • CO2 now present and detected in exhaled air Alveoli Capnogram Phase II Ascending Phase C Ascending II Phase Early A B Exhalation CO2 present and increasing in exhaled air Capnogram Phase III Alveolar Plateau • CO2 rich alveolar gas now constitutes the majority of the exhaled air • Uniform concentration of CO2 from alveoli to nose/mouth Capnogram Phase III Alveolar Plateau Alveolar Plateau CD III AB CO2 exhalation wave plateaus Capnogram Phase III End-Tidal • End of exhalation contains the highest concentration of CO2 – The “end-tidal CO2” – The number seen on your monitor • Normal EtCO2 is 35-45mmHg Capnogram Phase III End-Tidal End-tidal C D AB End of the the wave of exhalation Capnogram Phase IV Descending Phase • Inhalation begins • Oxygen fills airway • CO2 level quickly -

Clinical Update
Summer 2016 Clinical Update We are pleased to offer this archive of our award-winning newsletter Clinical Update. There are 75 issues in this document. Each issue has a feature article, summaries of articles in the nursing literature, and Web sites of interest. By downloading and using this archive, you agree that older medical articles may no longer describe appropriate practice. The issues are organized in date order from most recent to oldest. The following pages offer tips on how to navigate the issues and search the archive in Adobe Acrobat Reader. In 2006, we were honored to receive the Will Solimine Award of Excellence in Medical Writing from the American Medical Writers Association, New England Chapter. Issues that received the most positive response over the years include: • Nurses Removing Chest Tubes, a discussion of state boards of nursing’s approaches to this extended practice for registered nurses • Medical Adhesive Safety, a review of guidelines published by the Wound, Ostomy and Continence Nurses Society, complete with original tables identifying characteristics of each type of medical tape and how tape components contribute to medical adhesive- related skin injury (MARSI) • Autotransfusion for Jehovah’s Witness Patients, an explanation of the Biblical origins of the reasons for refusing blood transfusion and how continuous autotransfusion may offer an option that is acceptable to members of the faith • Air Transport for Patients with Chest Tubes and Pneumothorax and Chest Drainage and Hyperbaric Medicine, in which each issue provides a thorough analysis of how pressure changes with altitude and with increased atmospheric pressure affect chest drainage and untreated pneumothorax • Age Appropriate Competencies: Caring for Children that describes developmental stages and strategies to deal with a child’s fears at each stage Creative Commons License This work is licensed under a Creative Author: Patricia Carroll RN-BC, RRT, MS Commons Attribution-NonCommercial- ShareAlike 4.0 International License. -

Monitoring Anesthetic Depth
ANESTHETIC MONITORING Lyon Lee DVM PhD DACVA MONITORING ANESTHETIC DEPTH • The central nervous system is progressively depressed under general anesthesia. • Different stages of anesthesia will accompany different physiological reflexes and responses (see table below, Guedel’s signs and stages). Table 1. Guedel’s (1937) Signs and Stages of Anesthesia based on ‘Ether’ anesthesia in cats. Stages Description 1 Inducement, excitement, pupils constricted, voluntary struggling Obtunded reflexes, pupil diameters start to dilate, still excited, 2 involuntary struggling 3 Planes There are three planes- light, medium, and deep More decreased reflexes, pupils constricted, brisk palpebral reflex, Light corneal reflex, absence of swallowing reflex, lacrimation still present, no involuntary muscle movement. Ideal plane for most invasive procedures, pupils dilated, loss of pain, Medium loss of palpebral reflex, corneal reflexes present. Respiratory depression, severe muscle relaxation, bradycardia, no Deep (early overdose) reflexes (palpebral, corneal), pupils dilated Very deep anesthesia. Respiration ceases, cardiovascular function 4 depresses and death ensues immediately. • Due to arrival of newer inhalation anesthetics and concurrent use of injectable anesthetics and neuromuscular blockers the above classic signs do not fit well in most circumstances. • Modern concept has two stages simply dividing it into ‘awake’ and ‘unconscious’. • One should recognize and familiarize the reflexes with different physiologic signs to avoid any untoward side effects and complications • The system must be continuously monitored, and not neglected in favor of other signs of anesthesia. • Take all the information into account, not just one sign of anesthetic depth. • A major problem faced by all anesthetists is to avoid both ‘too light’ anesthesia with the risk of sudden violent movement and the dangerous ‘too deep’ anesthesia stage. -

Air & Surface Transport Nurses Association Position Statement
Air & Surface Transport Nurses Association Position Statement Advanced Airway Management Background In the early 1970s, civilian flight nursing became a recognized nursing specialty and an integral element in the care of critically ill and injured patients. Advanced airway management is an essential procedure in meeting those patient care goals. The procedure can be performed safely and effectively by properly trained transport teams; however, it is not without risks and complications. Individual state Boards of Nursing regulate registered nursing licensure. ASTNA believes transport providers and teams must work collegially and collaboratively with these regulatory bodies. Registered nurses who perform advanced airway management provide care under the direction and protocols of their medical directors. In addition, Registered Nurses who perform advanced airway management skills must have comprehensive initial and ongoing education to optimize clinical knowledge, skill, and decision-making ability. Education programs teaching these advanced airway management skills should include at least the following components: • Comprehensive review of airway/respiratory anatomy and physiology • Basic airway skills and techniques • Clinical assessment skills to evaluate the need for escalated intervention • Extraglottic airway devices • Tracheal intubation using a variety of devices • Surgical airway techniques • Pharmacology and clinical application of sedative, analgesic, hypnotic, and neuromuscular blocking agents used in advanced airway management • Patient safety monitoring equipment, including continuous pulse oximetry, continuous heart rate, and continuous monitoring of waveform capnography • Teamwork and crisis resource management, as applied to the clinical environment both as team leaders and team members Clinical best practices evolve continuously.1 Transport programs performing advanced airway should adopt policies that ensure clinical education and practice components remain current. -

Respiratory Therapy Pocket Reference
Pulmonary Physiology Volume Control Pressure Control Pressure Support Respiratory Therapy “AC” Assist Control; AC-VC, ~CMV (controlled mandatory Measure of static lung compliance. If in AC-VC, perform a.k.a. a.k.a. AC-PC; Assist Control Pressure Control; ~CMV-PC a.k.a PS (~BiPAP). Spontaneous: Pressure-present inspiratory pause (when there is no flow, there is no effect ventilation = all modes with RR and fixed Ti) PPlateau of Resistance; Pplat@Palv); or set Pause Time ~0.5s; RR, Pinsp, PEEP, FiO2, Flow Trigger, rise time, I:E (set Pocket Reference RR, Vt, PEEP, FiO2, Flow Trigger, Flow pattern, I:E (either Settings Pinsp, PEEP, FiO2, Flow Trigger, Rise time Target: < 30, Optimal: ~ 25 Settings directly or by inspiratory time Ti) Settings directly or via peak flow, Ti settings) Decreasing Ramp (potentially more physiologic) PIP: Total inspiratory work by vent; Reflects resistance & - Decreasing Ramp (potentially more physiologic) Card design by Respiratory care providers from: Square wave/constant vs Decreasing Ramp (potentially Flow Determined by: 1) PS level, 2) R, Rise Time ( rise time ® PPeak inspiratory compliance; Normal ~20 cmH20 (@8cc/kg and adult ETT); - Peak Flow determined by 1) Pinsp level, 2) R, 3)Ti (shorter Flow more physiologic) ¯ peak flow and 3.) pt effort Resp failure 30-40 (low VT use); Concern if >40. Flow = more flow), 4) pressure rise time (¯ Rise Time ® Peak v 0.9 Flow), 5) pt effort ( effort ® peak flow) Pplat-PEEP: tidal stress (lung injury & mortality risk). Target Determined by set RR, Vt, & Flow Pattern (i.e. for any set I:E Determined by patient effort & flow termination (“Esens” – PDriving peak flow, Square (¯ Ti) & Ramp ( Ti); Normal Ti: 1-1.5s; see below “Breath Termination”) < 15 cmH2O. -

Value of Pulse Oximetry in Screening for Long-Term Oxygen Therapy Requirement
Copyright CERS Journals Ltd 1993 Eur Reaplr J, 1993, 6, 559-562 European Respiratory Journal Printed In UK • all rlghta reaerved ISSN 0903 • 1936 TECHNICAL NOTE Value of pulse oximetry In screening for long-term oxygen therapy requirement C.M. Roberts, J.R. Bugler, R. Melchor, M.R. Hetzel, S.G. Spire Value of pulse oximetry in screening for long-term oxygen therapy requirement. C.M. Dept of Thoracic Medicine, University Roberts, J.R. Bugler, R. Melcltor, M.R. Hetzel, S.G. Spiro. aRS Journals Ltd 1993. College Hospital, Gower Street, London, ABSTRACT: Pulse oximetry, combined with spirometry, was evaluated as a method UK. of selecting chronic obstructive pulmonary disease (COPD) out-patients requiring definitive arterial blood gas analysis for long-term oxygen therapy (LTO'I} assess· Correspondence: C.M. Roberts The Chest Clinic ment. A relatively blgb screening arterial oxygen saturation by pulse oximetry Whipps Cross Hospital (Sao ) level was set, In order to maximize sensitivity. London Ell Ail 113 COPD out-patients att.endlng the hospital clinic over a 6 month period UK wet-e screened. Sixty bad a forced expiratory volume In one second d.S I and 26 Keywords: Chronic obstructive pulmonary bad an Sao1 s92 %. These 26 underwent arterial blood gas analysis. Nine had an ) disease arterial oxygen tension <7 .3 kPa all with an arterial carbon dioxide tension (Paco1 >6 kPa. A further eight had a Pao <8 kPa. This produced a sensitivity or 100% hypoxaemia and speclftclty or 69% for oximetry fn the detectl.on of Pao <7.3 kPa detenn.lned by oxygen therapy 1 pulse oximetry direct arterial puncture and 100% and 86% respectively for detecting a Pao1 <8 kPa. -

An Introduction to Anaesthesia
What You Need to KNoW about An introduction to anaesthesia Introduction divided into three stages: induction, main- n Central neuraxial block, e.g. spinal or Anaesthetic experience in the undergradu- tenance and emergence. epidural (Figure 1 and Table 1). ate timetable is often very limited so it can In regional anaesthesia, nerve transmis- remain somewhat of a mysterious practice sion is blocked, and the patient may stay Components of a general well into specialist training. This introduc- awake or be sedated or anaesthetized dur- anaesthetic tion to the components of an anaesthetic ing a procedure. Techniques used include: A general anaesthetic always involves an will help readers to get more from clinical n Local anaesthetic field block hypnotic agent, usually an analgesic and attachments in surgery and anaesthetics or n Peripheral nerve block may also include muscle relaxation. The serve as an introduction to the topic for n Nerve plexus block combination is referred to as the ‘triad of novice or non-anaesthetists. anaesthesia’. Figure 1. Schematic vertical longitudinal section The relative importance of each com- Types and sites of anaesthesia of vertebral column and structures encountered ponent depends on surgical and patient The term anaesthesia comes from the when performing central neuraxial blocks. * factors: the intervention planned, site, Greek meaning loss of sensation. negative pressure space filled with fat and surgical access requirement and the Anaesthetic practice has evolved from a venous plexi. † extends to S2, containing degree of pain or stimulation anticipated. need for pain relief and altered conscious- arachnoid mater, CSF, pia mater, spinal cord The technique is tailored to the individu- ness to allow surgery. -

Nasal and Forehead Oximetry Study
ARTICLE IN PRESS Heart & Lung ■■ (2018) ■■–■■ Contents lists available at ScienceDirect Heart & Lung journal homepage: www.heartandlung.com Comparison of nasal and forehead oximetry accuracy and pressure injury in critically ill patients Marilyn Schallom, RN, PhD, CCNS a,*, Donna Prentice, RN, PhD(c), APRN-C a, Carrie Sona, RN, MSN, CCNS a, Cassandra Arroyo, MS, PhD a,b, John Mazuski, MD, PhD b a Barnes-Jewish Hospital, One Barnes-Jewish Hospital Plaza, St. Louis, MO 63110, USA b Washington University School of Medicine, 660 S Euclid Ave., St. Louis, MO 63110, USA ARTICLE INFO ABSTRACT Article history: Background: In critically ill patients, clinicians can have difficulty obtaining accurate oximetry measurements. Received 31 July 2017 Objective: To compare the accuracy of nasal alar and forehead sensor measurements and incidence of Accepted 18 December 2017 pressure injury. Available online Methods: 43 patients had forehead and nasal alar sensors applied. Arterial samples were obtained at 0, 24, and 120 hours. Oxygen saturations measured by co-oximetry were compared to sensor values. Skin Keywords: was assessed every 8 hours. Pulse oximetry Results: Oxygen saturations ranged from 69.8%-97.8%, with 18% of measures < 90%. Measurements were Pressure injury Critically ill within 3% of co-oximetry values for 54% of nasal alar compared to 35% of forehead measurements. Mea- Norepineprhine surement failures occurred in 6% for nasal alar and 22% for forehead. Three patients developed a pressure Oxygen injury with the nasal alar sensor and 13 patients developed a pressure injury with the forehead sensor (χ2 = 7.68; p = .006). Conclusions: In this group of patients with decreased perfusion, nasal alar sensors provided a potential alternative for continuous monitoring of oxygen saturation. -

Inpatient Sepsis Management
Inpatient Sepsis Management - Adult Page 1 of 6 Disclaimer: This algorithm has been developed for MD Anderson using a multidisciplinary approach considering circumstances particular to MD Anderson’s specific patient population, services and structure, and clinical information. This is not intended to replace the independent medical or professional judgment of physicians or other health care providers in the context of individual clinical circumstances to determine a patient's care. This algorithm should not be used to treat pregnant women. PRESENTATION EVALUATION TREATMENT Call Code Blue Team1 Patient exhibits at least two of the Yes following modified SIRS criteria: ● Temperature < 36 or > 38.4°C Is patient ● MERIT team1 to evaluate and notify Primary MD/APP STAT ● Heart rate > 110 bpm unresponsive? ● Prepare transfer to ICU See Page 2: Sepsis ● Respiratory rate > 24 bpm ● Assess for presence of infection (see Appendix A) Management ● WBC count < 3 or > 15 K/microliter ● Assess for organ dysfunction (see Appendix B) No Yes ● Primary team to consider goals of care discussion if appropriate Is 1,2 the patient ● Sepsis APP to notify unstable? Primary MD/APP 1,2 ● Sepsis APP to evaluate ● See Page 2: Sepsis No ● Assess for presence of infection (see Yes Management Appendix A) Sepsis? ● Assess for organ dysfunction (see ● For further work up, Appendix B) No initiate Early Sepsis Order Set ● Follow up evaluation by SIRS = systemic inflammatory response syndrome Primary Team APP = advanced practice provider 1 For patients in the Acute Cancer -

Adequate Interval for the Monitoring of Vital Signs During Endotracheal Intubation J.Y
Min et al. BMC Anesthesiology (2017) 17:110 DOI 10.1186/s12871-017-0399-y RESEARCHARTICLE Open Access Adequate interval for the monitoring of vital signs during endotracheal intubation J.Y. Min1, H.I. Kim1, S.J Park1, H. Lim2, J.H. Song2 and H. J. Byon1* Abstract Background: In the perioperative period, it may be inappropriate to monitor vital signs during endotracheal intubation using the same interval as during a hemodynamically stable period. The aim of the present study was to determine whether it is appropriate to use the same intervals used during the endotracheal intubation and stable periods to monitor vital signs of patients under general anesthesia. Methods: The mean arterial pressure (MAP) and heart rate (HR) were continuously measured during endotracheal intubation (15 min after intubation) and hemodynamically stable (15 min before skin incision) periods in 24 general anesthesia patients. Data was considered “unrecognized” when continuously measured values were 30% more or less than the monitored value measured at 5- or 2.5-min intervals. The incidence of unrecognized data during endotracheal intubation was compared to that during the hemodynamically stable period. Result: ThereweresignificantlymoreunrecognizedMAPdatameasured at 5-min intervals during endotracheal intubation than during the hemodynamically stable period (p value <0.05). However, there was no difference in the incidence of unrecognized MAP data at 2.5 min intervals or HR data at 5 or 2.5 min intervals between during the endotracheal intubation and hemodynamically stable periods. Conclusion: A 5-min interval throughout the operation period was not appropriate for monitoring vital signs. Therefore, , a 2.5-min interval is recommended for monitoring the MAP during endotracheal intubation. -
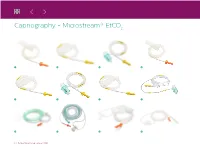
Capnography - Microstream® Etco2
Capnography - Microstream® EtCO2 10 11 12 6 | Patient Monitoring catalog 2016 Capnography - Microstream® EtCO2 Microstream® EtCO2 Solution Single Patient Use Product # Description Packaging Use with:* M1920A FilterLine Set, Adult/Pedi, Intubated M1921A FilterLine H Set, Adult/Pedi, Intubated M1923A FilterLine H Set, Infant/Neonatal, Intubated 989803160241 FilterLine® Set Long, Adult/Pediatric 25 sets 989803160251 FilterLine H Set Long, Adult/Pediatric Microstream® Ext: M3015A, M3015B, MP5: M8105A, 989803160261 FilterLine H Set Long, Infant/Neonatal M8105AS, VM8: 863066, VM1: 863266 989803159571 VitaLine™ H Set, Adult/Pediatric Efficia CM10 (863301), CM12 (863303), CM100 (863300), CM120 (863302), CM150 (863304) 989803159581 VitaLine™ H Set, Infant/Neonatal M2520A Smart CapnoLine® O2 Pediatric M2522A Smart CapnoLine® O2 Adult/Intermediate 25 FilterLines® 11 M2524A Smart CapnoLine® Pediatric M2526A Smart CapnoLine® Adult/Intermediate * Please reference the MySupplies or the e-store website for more details on hardware compatibility. Patient Monitoring catalog 2016 | 7 Capnography - Microstream® EtCO2 13 14 15 16 17 18 19 8 | Patient Monitoring catalog 2016 Capnography - Microstream® EtCO2 Microstream® EtCO2 Solution Single Patient Use (continued) Product # Description Reusable/ Single Patient Use Packaging Use with:* 13 989803160271 Smart CapnoLine® O2 Pediatric Long 989803160281 Smart CapnoLine® O2 Plus Adult Long 989803160301 Smart CapnoLine® Plus Adult Long 14 M4680A CapnoLine® H O2 Adult 15 M4681A CapnoLine® H O2 Pediatric 16 M4686A NIV