Detecting Emotional Response to Music Using Near-Infrared Spectroscopy of the Prefrontal Cortex
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Press Layout
2 Vol. XXXI, Issue 6 | Monday, November 23, 2009 news Southampton Doing What? meals, either. “The ladies yell at you for taking more, like if you try to take By Colleen Harrington an extra sandwich or something. I’m like, ‘I’m stocking up for when I miss Imagine shelling out hundreds of dinner!’” dollars for meals that you may never get For the more than a dozen stu- to eat. It’s not a charity or a food drive. dents, the experience was the same: It’s a fact of life for many resident stu- all had missed out on paid-for meals. dents at the Stony Brook Southampton “Seven o’clock is way too early to campus. close,” said Michael Geddes, a fresh- All students who live at the small man who’s studying sustainability at satellite campus are required to pur- Southampton. “There’s no where to chase one of three meal plans each se- go for food if you don’t have a car.” mester. Prices range from $1,739 to Marine vertebrate biology major $2,487. But there’s only one dining hall Joe Baillargeom, who reported miss- on campus, and it closes every night at ing meals a couple of times, said 7 p.m. Worse, there’s no breakfast on many of his fellow students complain weekends; instead, brunch begins at 11 about the dining hall hours, but just a.m. On the off-hours, hungry students deal with it. “Sometimes people will are stuck to choose from eating snacks make Easy Mac or Cup-of-Noodles out of campus vending machines, in the microwave downstairs, or hoarding groceries in their dormitories, they’ll just go off campus for food” or traveling off campus for food, despite when the café is closed, he said. -

Volume 45 - Issue 04 - Friday, October 2, 2009
Rose-Hulman Institute of Technology Rose-Hulman Scholar The Rose Thorn Archive Student Newspaper Fall 10-2-2009 Volume 45 - Issue 04 - Friday, October 2, 2009 Rose Thorn Staff Rose-Hulman Institute of Technology, [email protected] Follow this and additional works at: https://scholar.rose-hulman.edu/rosethorn Recommended Citation Rose Thorn Staff, "Volume 45 - Issue 04 - Friday, October 2, 2009" (2009). The Rose Thorn Archive. 106. https://scholar.rose-hulman.edu/rosethorn/106 THE MATERIAL POSTED ON THIS ROSE-HULMAN REPOSITORY IS TO BE USED FOR PRIVATE STUDY, SCHOLARSHIP, OR RESEARCH AND MAY NOT BE USED FOR ANY OTHER PURPOSE. SOME CONTENT IN THE MATERIAL POSTED ON THIS REPOSITORY MAY BE PROTECTED BY COPYRIGHT. ANYONE HAVING ACCESS TO THE MATERIAL SHOULD NOT REPRODUCE OR DISTRIBUTE BY ANY MEANS COPIES OF ANY OF THE MATERIAL OR USE THE MATERIAL FOR DIRECT OR INDIRECT COMMERCIAL ADVANTAGE WITHOUT DETERMINING THAT SUCH ACT OR ACTS WILL NOT INFRINGE THE COPYRIGHT RIGHTS OF ANY PERSON OR ENTITY. ANY REPRODUCTION OR DISTRIBUTION OF ANY MATERIAL POSTED ON THIS REPOSITORY IS AT THE SOLE RISK OF THE PARTY THAT DOES SO. This Book is brought to you for free and open access by the Student Newspaper at Rose-Hulman Scholar. It has been accepted for inclusion in The Rose Thorn Archive by an authorized administrator of Rose-Hulman Scholar. For more information, please contact [email protected]. T HE R OSE T HORN R OSE -H ULMAN I NSTITUTE OF T ECHNOLOGY T ERRE H AUTE , I NDIANA FRIDAY, OCTOBER 2, 2009 ROSE-HULMAN.EDU/THORN/ VOLUME 45, ISSUE 4 Homecoming 2009 News Briefs By Alex Mullans It was a culmination of a few Health care bill weeks of work and a long engi- discussed in earnest neering education condensed into just one night. -

Karaoke Mietsystem Songlist
Karaoke Mietsystem Songlist Ein Karaokesystem der Firma Showtronic Solutions AG in Zusammenarbeit mit Karafun. Karaoke-Katalog Update vom: 13/10/2020 Singen Sie online auf www.karafun.de Gesamter Katalog TOP 50 Shallow - A Star is Born Take Me Home, Country Roads - John Denver Skandal im Sperrbezirk - Spider Murphy Gang Griechischer Wein - Udo Jürgens Verdammt, Ich Lieb' Dich - Matthias Reim Dancing Queen - ABBA Dance Monkey - Tones and I Breaking Free - High School Musical In The Ghetto - Elvis Presley Angels - Robbie Williams Hulapalu - Andreas Gabalier Someone Like You - Adele 99 Luftballons - Nena Tage wie diese - Die Toten Hosen Ring of Fire - Johnny Cash Lemon Tree - Fool's Garden Ohne Dich (schlaf' ich heut' nacht nicht ein) - You Are the Reason - Calum Scott Perfect - Ed Sheeran Münchener Freiheit Stand by Me - Ben E. King Im Wagen Vor Mir - Henry Valentino And Uschi Let It Go - Idina Menzel Can You Feel The Love Tonight - The Lion King Atemlos durch die Nacht - Helene Fischer Roller - Apache 207 Someone You Loved - Lewis Capaldi I Want It That Way - Backstreet Boys Über Sieben Brücken Musst Du Gehn - Peter Maffay Summer Of '69 - Bryan Adams Cordula grün - Die Draufgänger Tequila - The Champs ...Baby One More Time - Britney Spears All of Me - John Legend Barbie Girl - Aqua Chasing Cars - Snow Patrol My Way - Frank Sinatra Hallelujah - Alexandra Burke Aber Bitte Mit Sahne - Udo Jürgens Bohemian Rhapsody - Queen Wannabe - Spice Girls Schrei nach Liebe - Die Ärzte Can't Help Falling In Love - Elvis Presley Country Roads - Hermes House Band Westerland - Die Ärzte Warum hast du nicht nein gesagt - Roland Kaiser Ich war noch niemals in New York - Ich War Noch Marmor, Stein Und Eisen Bricht - Drafi Deutscher Zombie - The Cranberries Niemals In New York Ich wollte nie erwachsen sein (Nessajas Lied) - Don't Stop Believing - Journey EXPLICIT Kann Texte enthalten, die nicht für Kinder und Jugendliche geeignet sind. -

Billboard Magazine
MUSIC ARTIST: AFI ALBUM: Burials RELEASE DATE: Oct. 22 LABEL: Republic PRODUCER: Gil Norton BAAUER 0 MANAGEMENT: Velvet Hammer Lucky Me/Mad Decent pro- Music and Management ducer Harry Bauer Rodrigues, Group better-known as Baauer, rose from unknown Brooklynite PUBLISHER: Ex Notcem to worldwide fame early Nacimur (bmi) this year when first single BOOKING AGENTS: Kirk “Harlem shake” became a Sommer and Dave Tamaroff, YouTube phenomenon. This William Morris Endeavor fall, he’s heading out on tour (international); emma Banks, with guests AraabMuzik, DJ Creative Artists Agency Mustard and S-Type, and (europe) hopes to release his debut al- bum in 2014. Aiding him with CHART HISTORY: Sing the his jaunt is AM Only agent Sorrow (2003), No. 5 De’mont Callender. Billboard 200, 1.3 million; Decemberunderground Routing: Rather than simply (2006), No. 1 billboard 200, 1 route Baauer across America, million; Crash Love (2009), No. Callender focused on smaller 12 billboard 200, 150,000 college towns with big audi- ences. “i wanted to hit a lot of TWITTER: @AFI the college markets where it’s in the middle of nowhere but has a huge college campus,” people as possible,” says David Benveniste, found- (Havok says that Blaqk Audio will return to the studio he says. “like the Knitting ROCK er/president of Velvet Hammer Music and Manage- once the Burials cycle wraps.) Factory in Boise, Idaho [Oct. 31] or the WOW Music Hall in ment Group. “There was a lot of excitement sur- “It was a really brief period of creation in relation to Eugene, Ore. [Oct. -

Voice of the Apaches
Voice of the Apaches Halloween 2009 WarCryNogales High School Volume XLI Issue II New honors coordinator prepared to help students By Ruth Portela Happy Haunting Nogales High School has a new Honors Coordinator The IB Diploma Programme enables students to be globally for this year. Mrs. Valenzuela is taking over this spot and recognized and well prepared for all aspects of life. The IB students making the best of it. She is an IB Chemistry teacher, strive to be inquirers, knowledgeable which she has been teaching for thinkers, communicators, principled, seven years, and this is her tenth open minded, caring, risk takers, year at NHS. balanced and reflective. “I enjoy teaching and I like “I expect to see more people to prepare students to the best of trying for the IB diploma. I also want my knowledge so they can go to to have more communication with college,” said Mrs. Valenzuela. As students in the community, and more an honors coordinator she has the parent involvement, as well as support responsibility to guide the honors towards the program from all NHS IB and AP students to achieve their staff.” goals. There is also the opening of the Mrs. Valenzuela feels she Honors Club and the purpose of this is fairly well-prepared for this club is to plan fundraiser activities to job. She has just finished a seven raise money for students to pay for the week online workshop which has exams. helped and answered many of her “I want to present all the information Who will win this year’s door decorating questions. -

Conscious Responsibility 2 Contents
Social Report 2016 World Without Barriers. VTB Group Conscious Responsibility 2 Contents VTB Group in 2016 4 3.3. Internal communications and corporate culture 50 Abbreviations 6 3.4. Occupational health and safety 53 Statement of the President and Chairman of the Management Board of VTB Bank (PJSC) 8 4. Social environment 56 1. About VTB Group 10 4.1. Development of the business environment 57 1.1. Group development strategy 12 4.2. Support for sports 63 1.2. CSR management 13 4.3. Support for culture and the arts 65 1.3. Internal (compliance) control 16 4.4. Support for health care and education 69 1.4. Stakeholder engagement 17 4.5. Support for vulnerable social groups 71 2. Market environment 26 5. Natural environment 74 2.1. Support for the public sector and socially significant business 27 5.1. Sustainable business practices 74 2.2. Support for SMBs 34 5.2. Financial support for environmental projects 77 2.3. Quality and access to banking services 35 6. About the Report 80 2.4. Socially significant retail services 38 7. Appendices 84 3. Internal environment 44 7.1. Membership in business associations 84 3.1. Employee training and development 46 7.2 GRI content index 86 3.2. Employee motivation and remuneration 49 7.3. Independent Assurance Report 90 VTB Social Report 2016 About VTB Group Market environment Internal environment Social environment Natural environment About the Report Appendices VTB Group in 2016 4 VTB Group in 2016 5 RUB 51.6 billion earned 72.7 thousand people 274.9 thousand MWh in net profit employed by the Group -

Love and Relationship Advice Award Season Recap Ehrow
MAGAZINE A VALENCIA VOICE PUBLICATION 2010 LOVE AND RELATIONSHIP ADVICE AWARD SEASON RECAP EHROW JEWLERY BY NICOLE Jewlery and Clothing line by Valencia’s very own students KID FAMOUS ENTERTAINMENT TABLE OF 6 Award show season wrap up 9 2010 movie preview 26 Kid Fmous FASHION/BEAUTY 34 Eli Louis 42 Jewlery by nicole 48 Etoile Health and Lifestyle 68 Health Tips 70Human Punching Bag LOVE AND RELATIONSHIP 62 First Date Mistakes 66 Crushin On You 35 Green Eyed Monster ACADEMICS 68 Summer School 70 Winter Dance CONTENTS 2 detour Hello Readers What is your stress reliever? Your break from ev- eryday life? For some its music and fashion. For others it may be family and friends. It is our pleasure to bring to you a brand new magazine from the Valencia Voice Publications. As a magazine dedicated to celebrating creative passion, we want you to take the journey with us as we experince the start of this exciting process. Detour Magazine was created as a way for students here at VCC or anywhere to just take a break from everyday life and take time to enjoy something that they’re passionate about. As a new magazine we want you to know that every reader is in our best interest. So what will Detour magazine cover you may ask? Well we have every- thing from what happend this awards season, to some of the best local designers found right here on campus. If you’re intrested in music don’t fret; we’ve got you covered ! We have worked very hard along our great and talented staff to bring you a magazine that will promise to keep interested from start to finish. -

Classical Folk & Blues Jazz Stage & Screen World Music
Fall, 2020 All Prices Good through 11/30/20 Music Classical see pages 3 - 24 Not Our First Goat Rodeo Yo-Yo Ma & Friends A classical-crossover selection SNYC 19439738552 $16.98 Folk & Blues see pages 42 - 49 Sierra Hull: 25 Trips Sierra jumps from her bluegrass roots to entirely new terrain 1CD# RDR 1166100579 $16.98 Jazz see pages 38 - 41 Diana Krall This Dream of You A wonderful collection of gems from the American Songbook 1CD# IMPU B003251902 $19.98 Stage & Screen see pages 25 - 29 Ennio Morricone Partnered with: Once Upon A Time Arrangements for Guitar 1CD# BLC 95855 $12.98 World Music see pages 34 - 37 Afwoyo – Afro Jazz Milégé derive inspiration from the diverse musical traditions of Uganda VT / KY / TN CD# NXS 76108 $16.98 see page 2 HBDirect Mixed-Genre Catalog HBDirect is pleased to present our newest Mixed-Genre catalog – bringing you the widest selection of classical, jazz, world music, folk, blues, band music, oldies and so much more! Call your Fall 2020 order in to our toll-free 800 line, mail it to us, or you can search for your selections on our website where you’ll also find thousands more recordings to choose from. Not Our First Goat Rodeo: Yo-Yo Ma & Friends Classical highlights in this issue include a tribute to Orfeo Records in honor of the label’s 40th Nine years ago, classical legends Yo Yo Ma, Stuart Duncan, Chris Thile, and anniversary, music of African American composers on page 8, plus new releases, videos, boxed sets Edgar Meyer released Goat Rodeo, and now they return with their masterful, and opera. -

Karaoke Catalog Updated On: 09/04/2018 Sing Online on Entire Catalog
Karaoke catalog Updated on: 09/04/2018 Sing online on www.karafun.com Entire catalog TOP 50 Tennessee Whiskey - Chris Stapleton My Way - Frank Sinatra Wannabe - Spice Girls Perfect - Ed Sheeran Take Me Home, Country Roads - John Denver Broken Halos - Chris Stapleton Sweet Caroline - Neil Diamond All Of Me - John Legend Sweet Child O'Mine - Guns N' Roses Don't Stop Believing - Journey Jackson - Johnny Cash Thinking Out Loud - Ed Sheeran Uptown Funk - Bruno Mars Wagon Wheel - Darius Rucker Neon Moon - Brooks & Dunn Friends In Low Places - Garth Brooks Fly Me To The Moon - Frank Sinatra Always On My Mind - Willie Nelson Girl Crush - Little Big Town Zombie - The Cranberries Ice Ice Baby - Vanilla Ice Folsom Prison Blues - Johnny Cash Piano Man - Billy Joel (Sittin' On) The Dock Of The Bay - Otis Redding Bohemian Rhapsody - Queen Turn The Page - Bob Seger Total Eclipse Of The Heart - Bonnie Tyler Ring Of Fire - Johnny Cash Me And Bobby McGee - Janis Joplin Man! I Feel Like A Woman! - Shania Twain Summer Nights - Grease House Of The Rising Sun - The Animals Strawberry Wine - Deana Carter Can't Help Falling In Love - Elvis Presley At Last - Etta James I Will Survive - Gloria Gaynor My Girl - The Temptations Killing Me Softly - The Fugees Jolene - Dolly Parton Before He Cheats - Carrie Underwood Amarillo By Morning - George Strait Love Shack - The B-52's Crazy - Patsy Cline I Want It That Way - Backstreet Boys In Case You Didn't Know - Brett Young Let It Go - Idina Menzel These Boots Are Made For Walkin' - Nancy Sinatra Livin' On A Prayer - Bon -

75 Years of Live Music in the Park CELEBRATE FOUR DECADES OF
FOR IMMEDIATE RELEASE CONTACT: Christine Reimert / 610‐639‐2136 / [email protected] Lucy MacNichol / 215‐568‐2525 / [email protected] Corey Bonser / 215‐546‐7900 / [email protected] 75 Years of Live Music in the Park CELEBRATE FOUR DECADES OF SONG WITH JULIO IGLESIAS ON A MAGICAL, STARRY NIGHT AT THE MANN Thursday, July 15th, 2010, 8 p.m. Tickets On Sale Now PHILADELPHIA (June 23, 2010) – Tickets are on sale now to experience the sounds of Julio Iglesias on his “Starry Night World Tour”, during his exclusive Philadelphia stop at The Mann Center for the Performing Arts. This concert at The Mann Center will focus on the emblematic songs from Iglesias’s four‐decade career. Part of a “no‐borders” world tour that has been already taken Iglesias to four continents (Oceania, Asia, America and Africa) and will soon bring him to Europe, this concert is dedicated to his fans and the celebration of his 42‐year, joyous career. Named for his third English album, the “Starry Night Tour” will feature songs like “Hey!”, “To All the Girls I´ve Loved Before”, “All of You”, “La cumparsita”, “Nathalie”, “A Media Luz”, “Abrázame”, and “Crazy.” Julio Iglesias Julio Iglesias has sold 300 million copies of his 79 albums released worldwide, including original versions in various languages, compilations, and live albums, which makes him the Spanish‐speaking artist who has sold the most albums in history. Julio Iglesias has sung duets with famous artists such as Frank Sinatra, Stevie Wonder, Willie Nelson, Diana Ross, Dolly Parton, Art Gurfunkel, Paul Anka, Charles Aznavour, Plácido Domingo, Sting, The Beach Boys, Nana Mouskouri, Mireille Mathieu, Dalida, Lola Flores, Pedro Vargas, Alejandro Fernández, José Luis Rodríguez, Wei Wei, Zeze di Camargo and Luciano. -

Le Merveilleux Petit Monde De Mireille Mathieu Chante Noel Mp3, Flac, Wma
Mireile Mathieu Le Merveilleux Petit Monde De Mireille Mathieu Chante Noel mp3, flac, wma DOWNLOAD LINKS (Clickable) Genre: Folk, World, & Country Album: Le Merveilleux Petit Monde De Mireille Mathieu Chante Noel Country: France MP3 version RAR size: 1486 mb FLAC version RAR size: 1634 mb WMA version RAR size: 1167 mb Rating: 4.6 Votes: 832 Other Formats: MP1 XM TTA WAV MIDI DMF AU Tracklist A1 White Christmas 3:42 A2 Petit Papa Noel 2:52 A3 Noel D'aubervilliers 2:58 A4 La Cambo Mi Fa Mauo 2:18 A5 Mon Beau Sapin 2:10 A6 Il Est Ne Le Divin Enfant 3:15 B1 Vive Le Vent 2:18 B2 Douce Nuit 3:10 B3 Rin Rin 2:21 B4 Les Enfants De Noel 2:34 B4 Minuit Chretiens 3:26 B5 Les Anges Dans Nos Campagnes 3:10 Other versions Category Artist Title (Format) Label Category Country Year Le Merveilleux Petit 80.376, 80 Mireille Monde De Mireille Barclay, 80.376, 80 France 1968 376 Mathieu Mathieu Chante Noel Barclay 376 (LP, Album) Mireille Mathieu Mireille 9101 701 Chante Noël (LP, Philips 9101 701 France Unknown Mathieu Album, RE, Gat) Mireille Mireille Mathieu, Mathieu, Großes Orchester Paul 79405 IU Großes Mariat* - Weihnachten Ariola 79405 IU Germany Unknown Orchester mit Mireille Mathieu Paul Mariat* (LP, Album) Mireille Mireille Mathieu's Capitol ST-490 ST-490 US Unknown Mathieu Christmas (LP) Records Mireille Chante Noel (LP, Overseas UPS-177-Y UPS-177-Y Japan 1972 Mathieu Album) Records Related Music albums to Le Merveilleux Petit Monde De Mireille Mathieu Chante Noel by Mireile Mathieu Mireille Mathieu - Rencontres De Femmes Mireille Mathieu - Je Vous Aime.. -
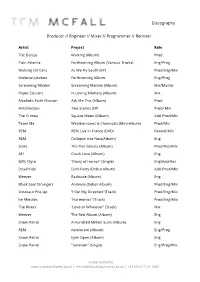
Tom Mcfall Discography
Discography Producer // Engineer // Mixer // Programmer // Remixer Artist Project Role The Darcys Warring (Album) Prod Twin Atlantic Forthcoming Album (Various Tracks) Eng/Prog Walking On Cars As We Fly South (EP) Prod/Eng/Mix Molotov Jukebox Forthcoming Album Eng/Prog Screaming Maldini Screaming Maldini (Album) Mix/Master Paper Scissors In Loving Memory (Album) Mix Alcoholic Faith Mission Ask Me This (Album) Prod Misdirectors Two Stones (EP) Prod/ Mix The Crimea Square Moon (Album) Add-Prod/Mix Team Me Weathervanes & Chemicals (Mini-Album) Prod/Mix REM REM Live in France (DVD) Record/Mix REM Collapse into Now(Album) Eng Stars The Five Ghosts (Album) Prod/Rec/Mix AFI Crash Love (Album) Eng Biffy Clyro “Many of Horror” (Single) Eng/Add-Rec Dead Kids Dark Party (Debut Album) Add-Prod/Mix Weezer Raditude (Album) Eng Black Soul Strangers Animate (Debut Album) Prod/Eng/Mix Dinosaur Pile-Up “I Get My Direction”(Track) Prod/Eng/Mix he Minutes “Harmonics” (Track) Prod/Eng/Mix The Rivers “Love or Whatever” (Track) Mix Weezer The Red Album (Album) Eng Snow Patrol A Hundred Million Suns (Album) Eng REM Accelerate (Album) Eng/Prog Snow Patrol Eyes Open (Album) Eng Snow Patrol "Isolation" (Single) Eng/Prog/Mix Audio Authority www.audioauthority.co.uk | [email protected] | +44 (0)20 7101 2880 Bloc Party Weekend In The City (Album) Eng Editors An End Has A Start (Album) Eng Stars Set Yourself on Fire (Album) Prod/Eng/Mix Vega 4 Various Tracks Eng/Prog/Mix Kill Kenada "Red and Black" (Single) Prod/Eng/Mix Television Room “Modern Boys & Girls”