Coliform Content of Shellfish (Anadara Antiquata) in Davao Gulf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
DAVAO CENTER for HEALTH DEVELOPMENT Os
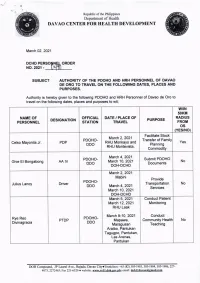
Republic of the Philippines /O" O\ Department of Health o DAVAO CENTER FOR HEALTH DEVELOPMENT Match 02,2021 DCHD PERSO N ORDER NO.202t - SUBJECT AUTHORITY OF THE POOHO AND HRH PERSONNEL OF DAVAO DE ORO TO TRAVEL ON THE FOLLOYUNG DATES, PLACES AND PURPOSES. Authority is hereby given to the following PDOHO and HRH Personnel of Davao de Oro to travel on the following dates, places and purposes to wit; wflN 50KU NATUIE OF OFFICIAL DATE / PLACE OF RADlUS DESIGNATION PURPOSE PERSONNEL STATION TRAVEL FROT os (YES/NO) Facilitate Stock March2,2021 PDOHO- Transfer of Family Celso Mayonila Jr. PDP RHU Monkayo and Yes DDO Planning RHU Montevista, Commodity March 4,2Q21 PDOHO- Submit PDOHO Ghie El Bongabong AA IV March 10, 2021 No DDO Documents DOH-DCHD March 2,2021 Mabini Provide PDOHO. Julius Lanoy Driver Transportation No DDO March 4,2021 Services March'10, 2021 DOH-DCHD March 5, 2021 Conduct Patient March 12,2021 Monitoring RHU Laak March 9-10, 2021 Conduct Rye Reo PDOHO- PTDP Mapawa, Community Health No Divinagracia DDO Maragusan Teaching Araibo, Pantukan Tagugpo, Pantukan, Las Arenas, Pantukan DOH Compound, JP laurel Ave., Bajada Davao City.Trunklines: +63 (E2) 305-1903, 305-1904, 305'1906,22'l- 4073,2272463iFu.221{320 o website: g44g4q[!!9[.ggy,p!; email: dg.b!149r!@4Ed!g, Republic of the Philippines .a" D. Department of Health .E:; o DAVAO CENTER FOR HEALTH DEVELOPIVTENT Conduct Technical Assistance regarding COVID 19 Reporting of Newly Hired PDOHO- March 9-10, 2021 Jason Mayang DSO Encoder No DDO RHU Maragusan Surveillance Functionality Assessment -

From Ideas to Action: a Review of Implementing HELP Principles in River Basins with Limited Resources and Capacity
From ideas to action: A review of implementing HELP principles in river basins with limited resources and capacity# Declan Hearne1* , Ruth Gamboa2 and Verna Marie Monsanto2 1 HELP Davao, 248 Arayat St., Central Park Phase I, Bangkal, Davao City, Philippines 2 The University of Philippines, Davao, Mindanao, Philippines Abstract Davao City is overlapped by 8 watersheds all flowing into the Davao Gulf. These watersheds exemplify a range of cultural, environmental and economic stresses from the continual conversion of natural habitat for agricultural, urban, and industrial uses. These changes and their consequent pressures have not gone unnoticed and have surfaced through various channels into the political and public arenas. However, despite the awareness and actions from various sectoral initiatives, there is continual deterioration of trends across the ecosystems. Hydrology for Environment, Life and Policy (HELP) is a global initiative which encourages policy makers, water man- agers, scientists, and end-users to work together within a field-oriented context to closely integrate science into government policies and management strategies. Through Davao City’s participation in the HELP Network, the management of water- sheds and water has improved not through the acquisition of additional external funds but by the increase in opportunity for dialogue between ‘water-related’ communities, which resulted to enhanced stakeholder understanding of issues and increased technical capacity of all involved. This paper demonstrates how the HELP principles can be applied in basins with limited resources and how these can posi- tively influence the attitudes and behaviour of stakeholders. It highlights how HELP can act as a catalyst to motivate learning, engage competing sectors, and build collaboration to create science-backed frameworks for good water governance. -

Coastal Environmental Profile of the Malalag Bay Area Davao Del Sur, Philippines
i COASTAL ENVIRONMENTAL PROFILE OF THE MALALAG BAY AREA DAVAO DEL SUR, PHILIPPINES IMELDA S. VALLE MA. CHONA B. CRISTOBAL ALAN T. WHITE EVELYN DEGUIT Coastal Resource Management Project of the Department of Environment and Natural Resources supported by the United States Agency for International Development 2000 ii Coastal Environmental Profile of the Malalag Bay Area, Davao del Sur, Philippines Imelda S. Valle, Ma. Chona B. Cristobal, Alan T. White and Evelyn T. Deguit 2000 PRINTED IN CEBU CITY, PHILIPPINES Citation: Valle, I.S., M.C.B. Cristobal, A.T. White and E. Deguit. 2000. Coastal Environmental Profile of the Malalag Bay Area, Davao del Sur, Philippines. Coastal Resource Management Project, Cebu City, Philippines, 127 p. This publication was made possible through support provided by the United States Agency for International Development (USAID) under the terms and conditions of Contract No. AID-492-C-00-96-00028-00 supporting the Coastal Resource Management Project (CRMP). The opinions expressed herein are those of the authors and do not necessarily reflect the views of the USAID. This publication may be reproduced or quoted in other publications as long as proper reference is made to the source. Production: Letty Dizon copy-edited and Lume Inamac and Ida Juliano word-processed, did the layout, and designed the cover of this document. Cover Photos: Front - A. White; Back - CRMP staff. CRMP Document No. 23-CRM/2000 ISBN 971-92289-9-7 iii CONTENTS Tables and Figures v Foreword vii Preface viii Acknowledgments x Acronyms and Abbreviations xi Commonly Used Local Terms xii Glossary of Terms xiv Chapter 1 Introduction 1 Chapter 2 Physical Features 7 Land Area 7 Topography 7 Hydrology 9 Soil 14 Land Uses 14 Climate 16 Chapter 3 Natural Resources 17 Mineral Resources 17 Forest Resources 17 Coastal Resources 18 Hagonoy 20 Malalag 23 Padada 26 Sta. -

Chapter 5 Improved Infrastructure and Logistics Support
Chapter 5 Improved Infrastructure and Logistics Support I. REGIONAL DEVELOPMENT CHALLENGES AND OPPORTUNITIES Davao Region still needs to improve its infrastructure facilities and services. While the Davao International Airport has been recently completed, road infrastructure, seaport, and telecommunication facilities need to be upgraded. Flood control and similar structures are needed in flood prone areas while power and water supply facilities are still lacking in the region’s remote and underserved areas. While the region is pushing for increased production of staple crops, irrigation support facilities in major agricultural production areas are still inadequate. Off-site infrastructure in designated tourism and agri-industrial areas are likewise needed to encourage investment and spur economic activities. Accessibility and Mobility through Transport There is a need for the construction of new roads and improvement of the existing road network to provide better access and linkage within and outside the Region as an alternate to existing arterial and local roads. The lack of good roads in the interior parts of the municipalities and provinces connecting to major arterial roads constrains the growth of agriculture and industry in the Region; it also limits the operations of transport services due to high maintenance cost and longer turnaround time. Traffic congestion is likewise becoming a problem in highly urbanized and urbanizing areas like Davao City and Tagum City. While the Region is physically connected with the adjoining regions in Mindanao, poor road condition in some major highways also hampers inter-regional economic activities. The expansion of agricultural activities in the resettlement and key production areas necessitates the opening and construction of alternative routes and farm-to-market roads. -

Ccn Tin Importer Im0006021794 430968150000 Daesang Ricor Corporation Im0002959372 003873536000 Westpoint Industrial Sales Co
CCN TIN IMPORTER IM0006021794 430968150000 DAESANG RICOR CORPORATION IM0002959372 003873536000 WESTPOINT INDUSTRIAL SALES CO. INC. IM0002992817 000695510000 ASIAN CARMAKERS CORPORATION IM0002963779 232347770000 STRONG LINK DEVELOPMENT CORPORATION IM0003299511 002624091000 TABAQUERIA DE FILIPINAS INC. IM0003063011 217711150000 ASIAWIDE REFRESHMENTS CORPORATION IM0002963639 001007787000 GX INTERNATIONAL INC. IM0006830714 456650820000 MOBIATRIX INC IM0003014592 002765139000 INNOVISTA TECHNOLOGIES INC. IM0003214699 005393872000 MONTEORO CHEMICAL CORPORATION IM0004340299 000126640000 LINKWORTH INTERNATIONAL INC. IM0006804179 417272052000 EATON INDUSTRIES PHILIPPINES LLC PH IM0002957590 000419293000 ALLEGRO MICROSYSTEMS PHILS. INC. IM0004143132 001030408000 PUENTESPINA ORCHIDS AND TROPICAL IM0003131297 004558769000 ARCHITECKS METAL SYSTEMS INC. IM0003025799 103873913000 MCMASTER INTERNATIONAL SALES IM0002973979 000296020000 CARE PRODUCTS INC IM0003014231 001026198000 INFRATEX PHILIPPINES INC. IM0002962691 000288655000 EURO-MED LABORATORIES PHILS. INC. IM0003031438 006818264000 NORTHFIELDS ENTERPRISES INT'L. INC. IM0003170217 002925850000 KENRICH INT'L . DISTRIBUTOR INC. IM0003259994 000365522000 KAMPILAN MANUFACTURING CORPORATION IM0003132498 103901522000 PEONY MERCHANDISING IM0002959496 204366533000 GLOBEWIDE TRADING IM0002966514 000070213000 NORKIS TRADING CO INC. IM0003232492 000117630000 ENERGIZER PHILIPPINES INC. IM0003131513 000319974000 HI-Q COMMERCIAL.INC IM0003035816 000237662000 PHILIPPINE INTERNATIONAL DEV'T INC. IM0003090795 113041122000 -

PROVINCIAL FISHERIES PROFILE Province of Compostela Valley
PROVINCIAL FISHERIES PROFILE Province of Compostela Valley I. GENERAL INFRORMATION** Total Land Area No.of Coastal Coastal Municipality Population (source No.of Barangay PPDO,Comval) (sq.km.) Barangay Population District 1 Maragusan 55,503 394.29 24 New Bataan 47,470 688.60 16 Compostela 81,934 187.50 16 Montevista 39,602 265.0 20 Monkayo 94,627 692.89 21 District 11 Nabunturan 73,196 245.29 28 Mawab 35,698 169.52 11 Laak 70,856 947.06 40 Maco 72,235 244.40 37 7 18,680 Mabini 36,807 412.25 11 6 20,286 Pantukan 79,067 420.13 13 9 51,352 TOTAL 687,195 4,666.93 237 22 90,318 II. FISHERY RESOURCES A. COMMERCIAL FISHERIES a. No.of Extension Worker Assigned……………………None b. No. of Commercial Fishing Vessel ( Catchers)……….None c. No. of Commercial Fishing Vessel (Registered)………None d. Total Annual Production (M.T./yr)……………………None e. No. of Licensed Commercial Fishermen………………None B. MUNICIPAL FISHERIES B.1 CAPTURE MUNICIPAL ** Municipality No.of Extension No.of No.of No.of Total No. of Total No.of Total Workers Assigned Fishermen Fishermen Motorized Annual non- Annual Fish Annual (Full time) (Part Banca Production Motorized Production Coral Production time) (M.T./Yr.) Banca (M.T./Yr.) (M.T./Yr.) Maco 2 (Alix Bellena & 180 115 150 200 122 95 3 1.5 Hanida Fernandez Mabini 2 (Amalia C. Bama 200 91 135 100 85 25 21 8 & Godofredo Fulo) Pantukan 2 (Bernardo G. Saga 456 142 410 775.79 188 180.55 9 2.84 & Lominicop Mambering TOTAL 836 348 695 1,145.79 395 365.55 33 12.34 B.2 INLAND MUNICIPAL *** Municipality Lake (ha.) River (ha.) Dams (ha.) SWIP (ha.) -

A Case Study on Indigenous Herbal Medicines
Ethnomedical documentation of and community health education for selected Philippine ethnolinguistic groups: the Mansaka people of Pantukan and Maragusan Valley, Compostela Valley Province, Mindanao, Philippines A collaborative project of Philippine Institute of Traditional and Alternative Health Care, Department of Health, Sta Cruz, Manila University of the Philippines Manila, Ermita, Manila University of the Philippines Mindanao, Bago Oshiro, Davao City 2000 TABLE OF CONTENTS Page Executive summary 1 Introduction 2 Objectives 3 Methodology 4 Results and discussion 7 Recommendations 21 References 22 Appendices 23 Tables Table 1. Materia medica Table 2. Mansaka terms Table 3. Mansaka demography ACKNOWLEDGEMENT We wish to acknowledge and express our heartfelt gratitude to the following people and organizations who have been significant partners in the undertaking of this research study: UP Mindanao Planning and Development Office for guidance and monitoring of the project; Mayor Jovito Derla and Municipal Assistant Administrator Mr Romeo Ang of Pantukan for permitting the research and helping in the selection of site; Mayor Gerome Lamparas of Maragusan Valley for permitting the research studies in the area; Barangay Captain Felicisimo C Marbones, Barangay Councilor and Matikadung Laurencio Camana of Barangay Napnapan for permitting the research to be conducted in Napnapan, Pantukan, Compostela Valley Province; Informants, contacts and guides from Maragusan and Pantukan communities for the support and unselfish sharing of their indigenous knowledge; Researcher’s family, friends and loved ones for their spiritual support through prayers for the safety during the one-year conduct of the research; and Above all, the Almighty God, to Whom all things were made possible. EXECUTIVE SUMMARY An ethnopharmacological study of the Mansaka people in the municipalities of Pantukan and Maragusan Valley, Compostela Valley Province was conducted in June 1999 to May 2000. -

Davao City Fishery Profile
DAVAO CITY FISHERY PROFILE I. GENERAL INFORMATION Total Land Area : 244,000 hectares Total Population : 1.4 million Total Number of Household : Total Number of Coastal Barangays : 26 Coastal Barangays Total Number of Coastal Population : 490,000 Total Number of Fisherfolk : 8,610 Boundaries: South : Sta. Cruz, Davao Del Sur North : Panabo City, Davao Del Norte II. FISHERY RESOURCES a) Municipal Fisheries No. of Fishing Bancas Motorized : 406 Non-Motorized : 391 Fish Catch (MT) : 820.97 MT No. Municipal Fishermen : 8,610 Fishing Gears/Method used : Active/Passive Gears No. of Fish Corral : 8 Annual Production : 200 MT b) Brackish water Fishpond Total Area : 173 ha Average Production : 320 MT / year No. of Operators : 15 operators Species Cultured : Bangus : 10.8 MT / year c) Mari-culture Fish Cages Total Area : 1.5 ha No. of operators : 30 operators Cage Size : 10m x 10m Annual Production : 400 MT Species Cultured : Milkfish / Siganids Seaweeds Total Area : 3 ha No. of Operators : 45 operators Length : 100m /line Annual Production : 132 MT Wet, 18.9 MT Dried d) Freshwater Fishpond Total Area : 78 ha No. of Operators : 172 Species Cultured Tilapia : 1MT/ha Hito : 3.3MT/ha Pangasius : 2.4MT/ha e) Hatcheries / Nurseries No. of Hatcheries : 12 hatcheries Total Area : 4,000 sq m No. of Nurseries : 12 nurseries Total Area : 15 ha Total No. of Hatchery Operators : 12 operators Total No. of Nursery Operators : 12 operators Average Production per Cropping : 7,600 pcs f) Marine Protected Area (MPA) Total Area : 473 ha Total No. of Barangay with MPA : 4 barangays Total No. -

PHI-OCHA Logistics Map 04Dec2012
Philippines: TY Bopha (Pablo) Road Matrix l Mindanao Tubay Madrid Cortes 9°10'N Carmen Mindanao Cabadbaran City Lanuza Southern Philippines Tandag City l Region XIII Remedios T. Romualdez (Caraga) Magallanes Region X Region IX 9°N Tago ARMM Sibagat Region XI Carmen (Davao) l Bayabas Nasipit San Miguel l Butuan City Surigao Cagwait Region XII Magsaysay del Sur Buenavista l 8°50'N Agusan del Norte Marihatag Gingoog City l Bayugan City Misamis DAVAO CITY- BUTUAN ROAD Oriental Las Nieves San Agustin DAVAO CITY TAGUM CITY NABUNTURAN MONTEVISTA MONKAYO TRENTO SAN FRANS BUTUAN DAVAO CITY 60km/1hr Prosperidad TAGUM CITY 90km/2hr 30km/1hr NABUNTURAN MONTEVISTA 102km/2.5hr 42km/1.5hr 12km/15mns 8°40'N 120km/2.45hr 60km/1hr 30km/45mns. 18kms/15mns Claveria Lianga MONKAYO 142km/3hr 82km/2.5hr 52km/1.5hr 40km/1hr 22km/30mns Esperanza TRENTO SAN FRANCISCO 200km/4hr 140km/3 hr 110km/2.5hr 98km/2.hr 80km/1.45hr 58km/1.5hr BUTUAN 314km/6hr 254km/5hr 224km/4hr 212km/3.5hr 194km/3hr 172km/2.45hr 114km/2hr l Barobo l 8°30'N San Luis Hinatuan Agusan Tagbina del Sur San Francisco Talacogon Impasug-Ong Rosario 8°20'N La Paz l Malaybalay City l Bislig City Bunawan Loreto 8°10'N l DAVAO CITY TO - LORETO, AGUSAN DEL SUR ROAD DAVAO CITY TAGUM CITY NABUNTURAN TRENTO STA. JOSEFA VERUELA LORETO DAVAO CITY 60km/1hr Lingig TAGUM CITY Cabanglasan Trento 90km/2hr 30km/1hr NABUNTURAN Veruela Santa Josefa TRENTO 142km/3hr 82km/2.5hr 52km/1.5hr STA. -

NDRRMC Sitrep No.1 Re Landslide in Compostela Valley 23 April 2011
REPUBLIC OF THE PHILIPPINES National Disaster Risk Reduction and Management Center, Camp Gen. Emilio Aguinaldo, Quezon City, Philippines NDRRMC UPDATE SitRep No. 1 re Landslide Incident in Panganason-B, Brgy. Kingking, Pantukan, Compostela Valley Province (Region XI) Releasing Officer USEC BENITO T. RAMOS Executive Director, NDRRMC and Administrator, OCD DATE: 23 April 2011, 2:00 PM Source: OCRC/RDRRMC XI & DSWD I. SITUATION OVERVIEW Profile of the Incident • Due to heavy rains, a landslide incident occurred at about 2:30 AM, 22 April 2011 in a small scale mining area at Panganason-B, Brgy. Kingking, Pantukan, Compostela Valley Province, resulting to the entrapment of several miners and damaging numerous houses Casualties • Dead: 3 Name Age Address Cause of Death/Remarks 1 - Jun Rex Torrejos La Filipina, Tagum City Landslide (the same person as Junjun Torrejos/John 15 yrs. old Torrejos which was previously reported in missing 2 - unidentified persons (still for verification) • Injured: 10 Name Age Address Remarks 1. Lambert Detros 47 yrs. old Matiao, Pantukan 2. Zeffrey Tunday 15 yrs. old Matiao, Pantukan Brought to Pantukan 3. Jerry Costal 21 yrs. old Lasang, Davao City Emergency Hospital 4. Mike Saret 30 yrs. old Asuncion, Davao del Norte Already released from Island Garden, City of 5. Erwin Galorio 20 yrs. old Pantukan Emergency Samal Hospital Brought to Davao Medical 6. Rico Clase 29 yrs. old Caraga, Davao Oriental Center, Tagum City Severely wounded and was brought to Davao 7. Rebecca Recaplaza Pantukan Medical Center, Tagum City 8. Nicolas Recaplaza Pantukan Out-patient 9. Jonathan Bilan Pantukan 10. Joel Lapates Bukidnon Telefax: NDRRMC Opcen (+632) 911-1406; 912-2665; 912-5668; NDRRMC Secretariat (+632) 912-0441; 912-5947; Office of the Administrator, OCD (+632) 912-2424 Email: [email protected] Website: www.ndcc.gov.ph • Missing : 21 Name Address 1. -

Chapter 4 Safety in the Philippines
Table of Contents Chapter 1 Philippine Regions ...................................................................................................................................... Chapter 2 Philippine Visa............................................................................................................................................. Chapter 3 Philippine Culture........................................................................................................................................ Chapter 4 Safety in the Philippines.............................................................................................................................. Chapter 5 Health & Wellness in the Philippines........................................................................................................... Chapter 6 Philippines Transportation........................................................................................................................... Chapter 7 Philippines Dating – Marriage..................................................................................................................... Chapter 8 Making a Living (Working & Investing) .................................................................................................... Chapter 9 Philippine Real Estate.................................................................................................................................. Chapter 10 Retiring in the Philippines........................................................................................................................... -

The Country Report of the Republic of the Philippines: Technical Seminar on South China Sea Fisheries Resources
The country report of the Republic of the Philippines: Technical seminar on South China Sea fisheries resources Item Type book_section Publisher Japan International Cooperation Agency Download date 30/09/2021 10:06:36 Link to Item http://hdl.handle.net/1834/40440 3.3 Other areas catch rate in waters shallower than 50 meters which are 3.3.1 East Malaysia fairly well exploited, and with a potential yield of 3.0 tons An estimate of potential yield is made for demersal and per square nautical mile. semipelagic species only based on the results of a single Unless very efficient gear, such as pair trawling, can be demersal trawl survey in the coastal waters up to about 50 employed to exploit successfully this sparse resource it is meters. The estimate is 183,000 tons but is more likely to not expected that major fishery can be developed. be between 91,500 to 137,250 tons. The potential yield (b) East coast of West Malaysia and East Malaysia per square nautical mile of 10.6 tons is similar to that of The estimate of potential yield is comprehensively the east coast of West Malaysia, 10.3 tons. dealt with by Shindo (IPFC/72/19) and as the average 3.3.2 Deeper waters density is low, though in some areas it is higher than (a) West coast of West Malaysia others, the problem of developing major fisheries for these In waters deeper than 50 meters the average catch rate demersal fish stocks is similar to the one discussed above of about 92.0 kg per hour was lower, about 64% of the for the west coast of West Malaysia.