25 29 U), 2020 |FKI Tower & Conrad Seoul, Seoul, Ko
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

National Tsing Hua University Senior Vice President Da Hsuan Feng Is Invited to Serve on Elsevier’S Global Academic Executive Board
Press Release from National Tsing Hua University For information, please contact Sabrina Li, Division of Public Affairs [email protected] +886-3-516-2372 National Tsing Hua University Senior Vice President Da Hsuan Feng is invited to serve on Elsevier’s Global Academic Executive Board Hsinchu, 16, 2012. Da Hsuan Feng, Senior Vice President of Global Strategy, Development and Evaluation of National Tsing Hua University in Hsinchu, Taiwan, has been invited to be a member of Elsevier’s Academic Executive Advisory Board (AEAB.) Membership of this committee consists of Academic Executives of leading Global research institutions and organizations. As stated succinctly in the website of AEAB, “The intention is not to discuss products or to act as development partners, but to hold a forum for an international group of academic executives to discuss common concerns. We’re honoured to act as hosts and feel we are becoming a better partner to the scientific community through this experience.” “The fact that the mission of AEAB is to seek better understanding through communications between publishers and scientific community, this makes my representing National Tsing Hua University that much more a solemn and heavy responsibility,” said Dr. Feng. “With that in mind, having a number of Asians to sit “at the table of an international committee” to explore routes to shape the now fast changing relationship between publishers and research universities implies the fundamental importance of Taiwan universities in particular, Asia universities in general in the 21st century is blinking brightly on the Global research radar screen,” said Feng. Other members of the Board are Jordi Alberch Vié, Vice Chancellor of Research, Universidad de Barcelona – Spain, Yuichiro Anzai, ex-President, Executive Advisor for Academic Affairs. -

Radomir Tylecote
Ownership and Innovation in Chinese Solar Photovoltaic Firms: An Analysis of the Effects of State, Private, and Foreign Shareholding on Patenting Performance Radomir Tylecote Thesis submitted for the award of Doctor of Philosophy (Ph.D.) The copyright of this thesis rests with the author and is made available under a Creative Commons Attribution Non-Commercial No Derivatives licence. Researchers are free to copy, distribute or transmit the thesis on the condition that they attribute it, that they do not use it for commercial purposes and that they do not alter, transform or build upon it. For any reuse or redistribution, researchers must make clear to others the licence terms of this work. 1 Abstract This thesis is a quantitative study of the effect of ownership – by state, private and foreign shareholdings – on innovation by China’s solar photovoltaic (PV) firms. Using the country’s solar PV industry, I seek to explain the impact of proportions of these different types of shareholding (and within the state category shareholding by central, provincial, and municipal governments) on innovative capacity. This capacity is measured by firms’ rates and qualities of patenting. As Chinese economic growth falters, amid the “re-shoring” of certain manufacturing capabilities, the role of the state, and whether it is helping or hindering its economy’s – and Chinese firms’ – technological upgrading, is a vital question. This is particularly true in high-tech sectors, including solar PV, which the Chinese government deems essential for the country’s continuing economic growth. Through the solar PV industry, we investigate the role of the state, and how it is helping or hindering Chinese companies’ innovation. -
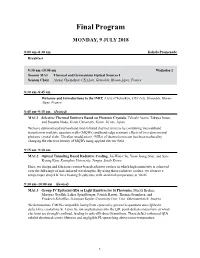
Final Program
Final Program MONDAY, 9 JULY 2018 8:00 am–8:30 am Kohala Promenade Breakfast 8:30 am–10:00 am Waikoloa 1 Session MA1 Thermal and Germanium Optical Sources I Session Chair Alexei Chelnokov, CEA Leti, Grenoble, Rhone-Alpes, France 8:30 am–8:45 am Welcome and Introductions to the IMIP, Alexei Chelnokov, CEA Leti, Grenoble, Rhone- Alpes, France 8:45 am–9:15 am (Invited) MA1.1 Selective Thermal Emitters Based on Photonic Crystals, Takashi Asano, Takuya Inoue, and Susumu Noda, Kyoto University, Kyoto, Kyoto, Japan We have demonstrated narrowband mid-infrared thermal emitters by combining intersubband transition in multiple quantum wells (MQWs) and band-edge resonant effects of two-dimensional photonic crystal slabs. Ultrafast modulation (~MHz) of thermal emission has been realized by changing the electron density of MQWs using applied electric field. 9:15 am–9:30 am MA1.2 Optical Tunneling Based Radiative Cooling, Jin-Woo Cho, Yoon Jeong Shin, and Sun- Kyung Kim, Kyunghee University, Yongin, South Korea Here, we design and fabricate cavities-based radiative coolers in which high emissivity is achieved over the full range of mid-infrared wavelengths. By using these radiative coolers, we observe a temperature drop 8 K for a heating Si substrate with an initial temperature at 340 K. 9:30 am–10:00 am (Invited) MA1.3 Group-IV Epitaxial QDs as Light Emitters for Si Photonics, Moritz Brehm, Martyna Grydlik, Lukas Spindlberger, Patrick Rauter, Thomas Fromherz, and Friedrich Schäffler, Johannes Kepler University Linz, Linz, Oberosterreich, Austria We demonstrate CMOS-compatible lasing from epitaxially-grown Ge quantum dots (QDs) in defect-free crystalline Si. -

Bibliographic Guide to the Literature
BIBLIOGRAPHIC GUIDE TO THE LITERATURE This bibliography combines and updates the bibliographies that appeared in Issues 1 and 2 of the China Environment Series. This information can also be viewed on the website of the Working Group on Environment in U.S.-China Relations at http://ecsp.si.edu/china-biblio.htm. The following entries are organized into eight sections: Agriculture; Biodiversity and Conservation; Climate Change; Energy; Environmental Management and Sustainable Development; Pollution and Health; Population and Urban Issues; and, Water. AGRICULTURE Lee, Yokshui F. “Rural Nonagricultural Activities in China: Assisting or Impeding Agriculture?” In, Develop- Beets, W.C., W.M. Rivera, R.C. Moore, Q.L. Yang, and ment or Deterioration? Work in Rural Asia, ed. Bruce Y. Hu. Mid-term Evaluation of Arid and Semi-Arid Agri- Koppel, John Hawkins, William James. Boulder: Lynne cultural Development in Northwest China. UNDP/Beijing Rienner Publishers, 1994. and UNDP/NY: Bureau for Asia and the Pacific, 1996. Parham, Walter E., Patricia J. Durana, and Alison Hess. Blobaum, Roger. “China Recycles Her Wastes by Using Improving Degraded Lands: Promising Experiences from Them on the Land.” Compost Science 5 (Autumn 1975): South China. Honolulu: Bishop Museum Press, 1993. 16-18. Pepall, Jennifer. “New Challenges for China’s Urban Brown, Lester R. Who Will Feed China? Wake-Up Call for Farmers.” IRDC Reports 3 (October 1993): 6-8. a Small Planet. New York: W.W. Norton, 1995. Prosterman, Roy, Tim Hanstad, and Li Ping. Large-Scale Cheng, Xu. “Sustainable Agricultural Development in Farming in China: An Appropriate Policy? Seattle: The China.” World Development 8 (August 1992): 1127-1144. -

China Environment Series 6
Foreword Jennifer L. Turner, Editor ver the past year, ECSP’s China Environment Forum held a diverse collection of meetings ranging from greening the 2008 Olympics to hazardous waste challenges in Greater China to U.S. assistance opportunities Oin the areas of environmental governance, rural health and environment problems, and coastal waters management. Summaries of all meetings are available in this issue of the China Environment Series and on our new Web site: www.wilsoncenter.org/cef. In addition to regular meetings in Washington, DC, we began a new initiative this year that has brought four U.S. and four Chinese water experts together to examine and compare water conflict resolution in the United States and China. The group met in Tucson, Arizona in February 2003 and will participate in a November 2003 study tour in China. A second China Environment Forum special initiative that will conclude in summer 2003 has brought U.S. and Chinese finance and environmental experts together to discuss how municipal bonds are created to build environmental infrastructure in the United States and whether such a system could be developed in China. For more details on these two study tour projects see the new Special Initiatives section in this issue of the China Environment Series. I was very pleased with the variety and depth of the feature articles in China Environment Series Issue 6—I learned so much from working with these authors. In the opening piece, Kelly Sims Gallagher presents the colorful modern history of how the Chinese government’s auto industry policies and patterns of international investment have helped to modernize the automobiles on China’s road today, but have not led to a high level of installed emissions control and fuel efficiency technology. -

Editorial Board
Editorial Board Guest Editorial Board Editors-in-Chief Hydro Projects Zhong, Zhihua, Chinese Academy of Engineering, China Guest Editors-in-Chief Reddy, Raj, Carnegie Mellon University, USA Aims & Scope Ma, Hongqi, Huaneng Lancang River Hydropower, Inc., China Executive Editor-in-Chief Berga, Luis, The Royal Academy of Sciences and Arts of Barcelona, Spain Schleiss, Anton, Swiss Committee on Dams, Switzerland Qian, Xuhong, East China University of Science and Technology, China Engineering is an international journal dedicated to the latest advancements in engineering. The goal of this journal is to provide a high-level platform Executive Editor-in-Chief Associate Editors-in-Chief where academic achievements of great importance in engineering science and technology can be disseminated and shared. Proceeding from scientific Jia, Jinsheng, China Institute of Water Resources and Hydropower Research, China Batterham, Robin, University of Melbourne, Australia discoveries, the research achievements disseminated by this journal should be convertible through innovation into a new productive force so as to Chen, Gang, Massachusetts Institute of Technology, USA Members Davis, Lance A. National Academy of Engineering, USA promote the development of world-class industries and engineering projects of great socio-economic significance. Suter, Ulrich W., Swiss Academy of Engineering Sciences, Switzerland Chen, Houqun, China Institute of Water Resources and Hydropower Research, China We are interested in: Tu, Hailing, General Research Institute for Nonferrous -

National Energy Futures Analysis and Energy Security Perspectives in China
National Energy Futures Analysis and Energy Security Perspectives in China --Strategic Thinking on the Energy issue in the 10th Five-Year Plan(FYP) Presentation for the Workshop on East Asia Energy Futures Ni Weidou, Li Zheng, Xue Yuan (Tsinghua University) Beijing, 2000.6 - 1 - Abstract A general introduction concerning the population, economics, industry structure and energy supply in China is presented in the paper, which points out that the present technology of energy is unsustainable for China from the point of view of resources and environment. When the perspective of supply of petroleum and natural gas is analyzed, the major problem of energy security in China is the shortage of liquid fuel. China is expecting a significant increase of natural gas production in recent years, but due to its limited share in the energy mix it cannot solve the energy security problem entirely. Some strategic thinking relating to the development of energy in 10th Five-Year Plan is raised. The development of fossil fuel fired power generation, hydroelectric power, nuclear power and renewable energy is analyzed, which leads to the urgent necessity of near term, intermediate term and long-term arrangement of clean coal utilization. The coal will still have the share in energy mix no less than 50% in the coming three or more decades. The polygeneration system based on oxygen blown coal gasification is emphasized in particular, as this system could provide super-clean energy for diverse sectors of industry, and provides advantages in resource optimization, economic benefits, and effective utilization of energy resulting in obvious environmental improvement. -

Optimizing Urban Development1 Shahid Yusuf and Kaoru Nabeshima
42485 Public Disclosure Authorized Public Disclosure Authorized Public Disclosure Authorized Public Disclosure Authorized DIRECTIONS INDEVELOPMENT Countries andRegions Consequences, Strategies, China Urbanizes Shahid YusufandTonySaich and Policies China Urbanizes China Urbanizes Consequences, Strategies, and Policies Shahid Yusuf Tony Saich © 2008 The International Bank for Reconstruction and Development / The World Bank 1818 H Street NW Washington DC 20433 Telephone: 202-473-1000 Internet: www.worldbank.org E-mail: [email protected] All rights reserved 1 2 3 4 10 09 08 07 This volume is a product of the staff of the International Bank for Reconstruction and Development / The World Bank. The findings, interpretations, and conclusions expressed in this volume do not necessarily reflect the views of the Executive Directors of The World Bank or the governments they represent. The World Bank does not guarantee the accuracy of the data included in this work. The bound- aries, colors, denominations, and other information shown on any map in this work do not imply any judgement on the part of The World Bank concerning the legal status of any territory or the endorsement or acceptance of such boundaries. Rights and Permissions The material in this publication is copyrighted. Copying and/or transmitting portions or all of this work without permission may be a violation of applicable law. The International Bank for Reconstruction and Development / The World Bank encourages dissemination of its work and will normally grant permission to reproduce portions of the work promptly. For permission to photocopy or reprint any part of this work, please send a request with com- plete information to the Copyright Clearance Center Inc., 222 Rosewood Drive, Danvers, MA 01923, USA; telephone: 978-750-8400; fax: 978-750-4470; Internet: www.copyright.com.