A Comparative Study of Complementary Feeding And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
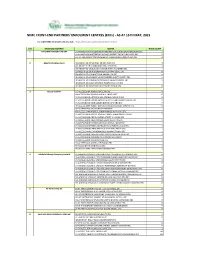
NIMC FRONT-END PARTNERS' ENROLMENT CENTRES (Ercs) - AS at 15TH MAY, 2021
NIMC FRONT-END PARTNERS' ENROLMENT CENTRES (ERCs) - AS AT 15TH MAY, 2021 For other NIMC enrolment centres, visit: https://nimc.gov.ng/nimc-enrolment-centres/ S/N FRONTEND PARTNER CENTER NODE COUNT 1 AA & MM MASTER FLAG ENT LA-AA AND MM MATSERFLAG AGBABIAKA STR ILOGBO EREMI BADAGRY ERC 1 LA-AA AND MM MATSERFLAG AGUMO MARKET OKOAFO BADAGRY ERC 0 OG-AA AND MM MATSERFLAG BAALE COMPOUND KOFEDOTI LGA ERC 0 2 Abuchi Ed.Ogbuju & Co AB-ABUCHI-ED ST MICHAEL RD ABA ABIA ERC 2 AN-ABUCHI-ED BUILDING MATERIAL OGIDI ERC 2 AN-ABUCHI-ED OGBUJU ZIK AVENUE AWKA ANAMBRA ERC 1 EB-ABUCHI-ED ENUGU BABAKALIKI EXP WAY ISIEKE ERC 0 EN-ABUCHI-ED UDUMA TOWN ANINRI LGA ERC 0 IM-ABUCHI-ED MBAKWE SQUARE ISIOKPO IDEATO NORTH ERC 1 IM-ABUCHI-ED UGBA AFOR OBOHIA RD AHIAZU MBAISE ERC 1 IM-ABUCHI-ED UGBA AMAIFEKE TOWN ORLU LGA ERC 1 IM-ABUCHI-ED UMUNEKE NGOR NGOR OKPALA ERC 0 3 Access Bank Plc DT-ACCESS BANK WARRI SAPELE RD ERC 0 EN-ACCESS BANK GARDEN AVENUE ENUGU ERC 0 FC-ACCESS BANK ADETOKUNBO ADEMOLA WUSE II ERC 0 FC-ACCESS BANK LADOKE AKINTOLA BOULEVARD GARKI II ABUJA ERC 1 FC-ACCESS BANK MOHAMMED BUHARI WAY CBD ERC 0 IM-ACCESS BANK WAAST AVENUE IKENEGBU LAYOUT OWERRI ERC 0 KD-ACCESS BANK KACHIA RD KADUNA ERC 1 KN-ACCESS BANK MURTALA MOHAMMED WAY KANO ERC 1 LA-ACCESS BANK ACCESS TOWERS PRINCE ALABA ONIRU STR ERC 1 LA-ACCESS BANK ADEOLA ODEKU STREET VI LAGOS ERC 1 LA-ACCESS BANK ADETOKUNBO ADEMOLA STR VI ERC 1 LA-ACCESS BANK IKOTUN JUNCTION IKOTUN LAGOS ERC 1 LA-ACCESS BANK ITIRE LAWANSON RD SURULERE LAGOS ERC 1 LA-ACCESS BANK LAGOS ABEOKUTA EXP WAY AGEGE ERC 1 LA-ACCESS -

African Journal of Pedagogy
ii African Journal of Pedagogy AFRICAN JOURNAL OF PEDAGOGY ISSN 1821-8474 VOLUME 6, JUNE, 2013 Volume 6, June, 2013 iii EDITORIAL BOARD Editor-in-Chief: Josephine Nassimbwa Associate Dean Faculty of Education Kampala International University College Dar es Salaam, Tanzania Consulting Editors 1) M.A. Ogunu Faculty of Education University of Benin, Nigeria. 2) Emmanuel Eneyo Southern Illinois University Edwardsville United States of America 3) Gabriel Olubunmi Alegbeleye Faculty of Education University of Ibadan, Nigeria. 4) Matthew Paris University Library Southern Illinois University Edwardsville United States of America 5) Mon Nwadiani Faculty of Education University of Benin, Nigeria. 6) Harry Akusah University of Ghana Legon, Accra Ghana iv African Journal of Pedagogy 7) Deola Omoba Oluwasanmi Hezekiah Library Obafemi Awolowo University Ile-Ife Nigeria 8) Johnson Dehinbo Faculty of Information & Communications Technology Tshwane University of Technology Soshanguve 0152 South Africa Volume 6, June, 2013 v African Journal of Pedagogy The African Journal of Pedagogy (AJEP) is a Tanzania-based journal that publishes high-quality solicited and unsolicited articles in all areas of education. Such articles must be written in good English language. Articles to be published in the African Journal of Pedagogy are usually subjected to peer-review. The African Journal of Pedagogy will be published yearly with effect from January, 2014. It was a quarterly publication. Types of articles that are acceptable The African Journal of Pedagogy accepts two forms of articles for publication. These are: 1) Report of empirical studies: These should describe new and carefully confirmed findings, and details of the research methods should be given so that others can verify the work. -

Download (7Mb)
\ Family Structure and Reproductive Health Decision Making among the Ogu of Southwestern Nigeria: A Qualitative Study Onipede WUSli Uche C. Isiugo-Abullilre Lagos State University University of lbadan Gjo Lagos, igeria lbadan, igeria Abstract Tilis study examini's the structure of the Ogu fall/Ily and its influcnce on reprodllcU,,1' IwaItl! decision-mJlking liSin a qualitative approach. Data 7Pcre sourced throllgh nine jC)CIIS groups orgmlized in the study am? Jll/lOng married lIlen and wOl1len. The data revml IIwl the family structllre in the study arm is changing, ,tlthough IIJe domillrlilt pailI'm remains extcnded. The findings or thi' sludy suggesl thai II ere are all-going illiemal trll/lsjorllwliol/s Ihat lend /0 wlll!J1ce gcnder equity in rcprodudive health decIsIOn-making !Jl!twcell hllshtmds lind wiui's. Tilesi' c/zrmges lIIay be attribu led to the widespread luflllence of western culture and the spread of edllcation in the sludy populalioll, which are necessary cOilcolllitmlls or eCO/lOIIlIC, polilicaland cll/tural changes laking pilice in the society. Resume Cet article examine la structure de 1(/ famille Ogu et SOli il~fluence sur III prise de dhisioll ell matiere de sallte de la reproduction. Les domll:es 1I1ilisces 50111 des dOlllleeS qualitatives obtenues ?i partil' de 9 "FoclIs Group" organises elltre homilies ct fe171mes mariees dll milieu d'Uude. Les do IIIIces /IIol1trelll que la slructrnc de la fall/ille dalls ce milieu d'etllde evo/lle biell ql/C Ie type dom/rumt reste la flll1lille elal'gle. Les resullllts de ['etude /!lemlre/lt 'Ilie des transformations inlemes I Ildant aproll/ollvoir /'egalitc entre les gellres ell malicre de pnse de decisioll cOllcemant III sallie de la reproductio/'! entre cpoux et ePOllSeS sonl enlmin de s'operer. -

Nigeria's Constitution of 1999
PDF generated: 26 Aug 2021, 16:42 constituteproject.org Nigeria's Constitution of 1999 This complete constitution has been generated from excerpts of texts from the repository of the Comparative Constitutions Project, and distributed on constituteproject.org. constituteproject.org PDF generated: 26 Aug 2021, 16:42 Table of contents Preamble . 5 Chapter I: General Provisions . 5 Part I: Federal Republic of Nigeria . 5 Part II: Powers of the Federal Republic of Nigeria . 6 Chapter II: Fundamental Objectives and Directive Principles of State Policy . 13 Chapter III: Citizenship . 17 Chapter IV: Fundamental Rights . 20 Chapter V: The Legislature . 28 Part I: National Assembly . 28 A. Composition and Staff of National Assembly . 28 B. Procedure for Summoning and Dissolution of National Assembly . 29 C. Qualifications for Membership of National Assembly and Right of Attendance . 32 D. Elections to National Assembly . 35 E. Powers and Control over Public Funds . 36 Part II: House of Assembly of a State . 40 A. Composition and Staff of House of Assembly . 40 B. Procedure for Summoning and Dissolution of House of Assembly . 41 C. Qualification for Membership of House of Assembly and Right of Attendance . 43 D. Elections to a House of Assembly . 45 E. Powers and Control over Public Funds . 47 Chapter VI: The Executive . 50 Part I: Federal Executive . 50 A. The President of the Federation . 50 B. Establishment of Certain Federal Executive Bodies . 58 C. Public Revenue . 61 D. The Public Service of the Federation . 63 Part II: State Executive . 65 A. Governor of a State . 65 B. Establishment of Certain State Executive Bodies . -

Effect of Health Education on Hiv Counselling and Testing (Hct) for Antenatal Clients in Abeokuta South Local Government Area of Ogun State, Nigeria
EFFECT OF HEALTH EDUCATION ON HIV COUNSELLING AND TESTING (HCT) FOR ANTENATAL CLIENTS IN ABEOKUTA SOUTH LOCAL GOVERNMENT AREA OF OGUN STATE, NIGERIA BY DR. OLURANTI. O. SOFOWORA BSC, MBBS, MPH, MPA. A DISSERTATION SUBMITTED TO THE NATIONAL POST GRADUATE MEDICAL COLLEGE OF NIGERIA IN PARTIAL FULFILMENT FOR THE AWARD OF PART II (FINAL) FELLOWSHIP IN THE FACULTY OF PUBLIC HEALTH (FMCPH) NOVEMBER 2009 CERTIFICATION We hereby certify that this research work titled: “Effect of health education on HIV Counselling and Testing (HCT) for HIV/AIDS among antenatal clients in 1 Abeokuta South Local Government Area of Ogun State, Nigeria” was carried out by Dr. Oluranti O. Sofowora of the Department of Community Medicine and Primary Care, Olabisi Onabanjo University Teaching Hospital, Sagamu under our supervision. Professor O.K. Alausa, FMCPH, FMCPath Supervisor Dr. F.A. Oluwole, FWACP Head of Department DECLARATION It is hereby declared that this work “Effect of health education on HIV counselling and testing (HCT) for HIV/AIDS among antenatal clients in Abeokuta South Local Government 2 Area of Ogun state, Nigeria” is original unless otherwise acknowledge. The work has not been presented to any other College, body or institution for a Fellowship or for any other publications or purposes. -------------------------------------------------- DR. OLURANTI .O. SOFOWORA DEDICATION To my Father, Almighty Father, He is King of Kings, 3 He is Lord of Lords. ACKNOWLEDGEMENTS Firstly, I wish to thank my Almighty Father in Heaven and the Lord Jesus Christ for being with me and granting me the grace, guidance and strength throughout my studies. I am greatly indebted to Prof. -

Historical Facts
HISTORICAL FACTS HOW GOO HAS LAGOS BEEN TO THE INDIGENES? Being a lecture presented by Habeeb Abiodun Sanni at the One any conference on political situation in Lagos State, organized by the committee of the Indigenous Association of Lagos State held at sycamore hotel, Ajara Badagry on Saturday 11, 2007. The Chairman of the organizing Committee, Hon, Justice S.O. Hunponu-Wasiu, Member of the committee, Your Royal Highness, leaders of the various indigenous Association of Lagos State here present, Indigenes of Lagos State, Members of the press, Ladies and gentlemen, all protocols duly observed. I am very delighted to be the guest speaker called upon by this body on the occasion of most competent person among the multitude of scholars devoted to teaching and researching on the whatever I discuss here today will not only be beneficial to all sundry, but could also serve as catalyst toward achieving the aims of the organizers’ dream of Lagos for the indigenes was akin to the basis upon which the United Muslim Party, separation of Lagos from the Western Region during the last decade of decolonization from Britain. The organizations such as F.R.A Williams, S.L. Akintola, and Chief Obafemi Awolowo, all of the Action Group, which preferred the retention of Lagos as part of the west. Supporting the position of the U.M.P, However, were Chief H.O Davies and Chief Odofin Akinyele. The latter founded the Lagos Regional Party, ostensibly to contest all local and general elections and use every means at their disposal to ensure that the separation of Lagos from the west was permanent and irrevocable. -

Lagos State Poctket Factfinder
HISTORY Before the creation of the States in 1967, the identity of Lagos was restricted to the Lagos Island of Eko (Bini word for war camp). The first settlers in Eko were the Aworis, who were mostly hunters and fishermen. They had migrated from Ile-Ife by stages to the coast at Ebute- Metta. The Aworis were later reinforced by a band of Benin warriors and joined by other Yoruba elements who settled on the mainland for a while till the danger of an attack by the warring tribes plaguing Yorubaland drove them to seek the security of the nearest island, Iddo, from where they spread to Eko. By 1851 after the abolition of the slave trade, there was a great attraction to Lagos by the repatriates. First were the Saro, mainly freed Yoruba captives and their descendants who, having been set ashore in Sierra Leone, responded to the pull of their homeland, and returned in successive waves to Lagos. Having had the privilege of Western education and christianity, they made remarkable contributions to education and the rapid modernisation of Lagos. They were granted land to settle in the Olowogbowo and Breadfruit areas of the island. The Brazilian returnees, the Aguda, also started arriving in Lagos in the mid-19th century and brought with them the skills they had acquired in Brazil. Most of them were master-builders, carpenters and masons, and gave the distinct charaterisitics of Brazilian architecture to their residential buildings at Bamgbose and Campos Square areas which form a large proportion of architectural richness of the city. -

Beach Tourism in Nigeria: a Case Study of Elegushi Beach Resort, Lagos State
BEACH TOURISM IN NIGERIA: A CASE STUDY OF ELEGUSHI BEACH RESORT, LAGOS STATE. BY OKOYE, CHINENYE N. PG/MA/12/62766 A PROJECT REPORT SUBMITTED TO THE DEPARTMENT OF ARCHAEOLOGY AND TOURISM, FACULTY OFARTS, UNIVERSITY OF NIGERIA, NSUKKA IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE AWARD OF MASTER OF ARTS DEGREE IN CULTURAL RESOURCE MANAGEMENTAND TOURISM FEBRUARY, 2014 i TITLE PAGE BEACH TOURISM IN NIGERIA: A CASE STUDY OF ELEGUSHI BEACH RESORT, LAGOS STATE i APPROVAL PAGE BEACH TOURISM IN NIGERIA: A CASE STUDY OF ELEGUSHI BEACH RESORT, LAGOS STATE BY OKOYE, CHINENYE N. PG/MA/12/62766 THIS PROJECT HAS BEEN APPROVED FOR THE DEPARTMENT OF ARCHAEOLOGY AND TOURISM, UNIVERSITY OF NIGERIA, NSUKKA. BY ______________________ ______________________ SUPERVISOR EXTERRNAL EXAMINER ______________________ _______________________ HEAD OF DEPARTMENT DEAN OF FACULTY ii CERTIFICATION OKOYE, CHINENYE NGOZI, a Post-graduate student in the Department of Archaeology/Tourism with Registration Number, PG/MA/12/62766, has satisfactorily completed the requirements for courses and the research work for the Master of Arts Degree in Cultural Resources Management and Tourism. The work embodied in this project report is original and has not been submitted in part or in full for any other diploma or degree of this or any other university. __________________________ __________________________ DR. L. C. EKECHUKWU PROF. E. E. OKAFOR (SUPERVISOR) (HEAD OF DEPARTMENT) _______________________________ EXTERNAL EXAMINER iii DEDICATION This project is dedicated to God Almighty and to my beloved parents (Mr. and Mrs. Emma Okoye). iv ACKNOWLEDGEMENTS To God be the glory for keeping me alive all through this programme and during my research study. -

Feasibility Study on the Import of Fresh Organic Coconut from Nigeria to Germany
FEASIBILITY STUDY ON THE IMPORT OF FRESH ORGANIC COCONUT FROM NIGERIA TO GERMANY. A CASE STUDY OF BIOTROPIC IMPORT COMPANY, GERMANY. A Research project Submitted to: Van Hall Larenstein University of Applied Sciences in Partial Fulfilment of the Requirements for the Degree of Master in Agricultural production chain management, Specialisation; Horticulture Chain. By Olabiran Olubunmi Bashirat September 2012. i Acknowledgement This research would not have been possible without the guidance and the help of several individuals who in one way or another contributed and extended their valuable assistance in the preparation and completion of this study. First and foremost, my utmost gratitude to my family for their moral and financial support as well as their dedication throughout the period of this research. I will also like to appreciate my supervisor, Jan Hoekstra for his concrete criticism and useful advices during the writing of this report. Not forgetting Tolu Fadesere, Seun fakeye and Rajathu for their assistance on the report writing. Finally and most importantly to God Almighty who made this research possible. ii Table of Contents Acknowledgement ........................................................................................................................ ii List of figures ................................................................................................................................. v List of tables ................................................................................................................................... -

MTN, CBN Near Truce on $8.1Bn Refund
businessday market monitor NSE Bitcoin E verdon Bureau De Change FMDQ Close FOREIGN EXCHANGE TREASURY BILLS FGN BONDS - $42.46bn Foreign Reserve Market Spot ($/N) 3M 6M 5 Y 10 Y 20 Y Biggest Gainer Biggest Loser BUY SELL Cross Rates - GBP-$:1.29 YUANY-N52.28 ₦2,284,871.90 +0.08 pc 0.06 -0.01 Guinness Nestle $-N 359.00 362.00 I&E FX Window 364.01 0.32 0.32 -0.04 Commodities 15.17 N80.5 1.26 pc N1370 -2.14 pc Powered by £-N 468.00 476.00 CBN Official Rate 306.55 13.21 13.36 15.10 15.39 Cocoa Gold Crude Oil 32,403.60 €-N 407.50 415.50 Currency Futures NGUS DEC 26 2018 NGUS MAR 27 2019 NGUS SEP 18 2019 US $2,139.00 $1,230.40 $77.17 ($/N) 364.27 364.72 365.62 NEWS YOU CAN TRUST I **THURSDAY 25 OCTOBER 2018 I VOL. 15, NO 169 I N300 @ g ‘Nigeria’s private sector has no FG to spend N8.73trn in 2019, capacity to fund infrastructure’ ONYINYE NWACHUKWU, Abuja igeria’s private sector targets 3.01% economic growth has no capacity to fund Nbig ticket infrastructure TONY AILEMEN, Abuja projects in the country, Baba- Leaves exchange rate unchanged at N305/$ tunde Fashola Minister of Power, he Federal Execu- Works and Housing, said at a tive Council (FEC), session on infrastructure at the Wednesday ap- Raises crude oil price benchmark to $60/b Nigeria Economic Summit. proved a total of This is despite the belief by N8.73 trillion budget Term Expenditure Framework Correspondents after the weekly sembly for approval. -

Drums As a Unifying Deity in Africa: Reminiscing the Nigerian Drum
International Journal of Applied Research 2018; 4(10): 343-348 ISSN Print: 2394-7500 ISSN Online: 2394-5869 Drums as a unifying deity in Africa: Reminiscing the Impact Factor: 5.2 IJAR 2018; 4(10): 343-348 Nigerian drum festival www.allresearchjournal.com Received: 11-08-2018 Accepted: 15-09-2018 Ovunda Ihunwo Ovunda Ihunwo Ph.D. Department of Theatre Abstract and Film Studies University of The idea that certain beliefs in Africa are fetish is not only demeaning but has the tendency of eroding Port Harcourt, Nigeria the very rich cultural heritage in the continent. Studies have shown that Africa is blessed with so many untold stories which have hitherto been hidden as a result of the inability of our forefathers to document events that have given rise to such mythology. Drumming as an art form is found to be a unifying tool that cut across different nationalities, yet have the semblance of a deified being. This paper unveils the important role the drum plays in the life of Africans using the Nigerian Drums Festival as a case in point. Method of research and information gathering are mostly from personal interviews with veteran drummers who participated in the Festival which held in Ogun State, Nigeria at the June 12 Cultural Centre on 22nd April, 2016. Findings have shown that Africans revere and deify certain drums as a result of the kind of roles they have played in sacred events in their communities. Keywords: drums, unifying deity, reminiscing, drum festival, Africa, Nigerian Introduction Drums are wonderful instruments that produce pleasant sounds for the purposes entertainment, rhythm or music. -

Trans-National Threats and National Security Concerns: a Study of Nigeria-Benin Republic Border
International Security Journal Number 1 Issue 1 ISSN 2045-2195 2011 Trans-national threats and national security concerns: A study of Nigerian-Benin Republic border Ngboawaji Daniel Nte Department of Social Studies River State University of Education Nigeria [email protected] Abstract This work is a research paper that tried to examine the trans-national threats posed by crimes across the Nigeria-Benin Republic border and the impacts on the national security of both countries. The study found out that such crimes as; smuggling, child and women trafficking, small and light weapon trafficking and trafficking of narcotics across the border. These crimes, the study notes pose serious threat to national security. The study which relied on primary and secondary data tested four hypotheses which gave useful guides to policy implications and recommendations that will help improve security at the Nigeria-Benin Republic border if adopted. KEY WORDS: Trans-national Threats, Security, Nigeria, Benin Republic, Globalisation, Crime. 1. Introduction While the international order among states is quite strong, the world is not peaceful. In the wake of globalization and the explosion in communication technologies, new security related threats have emerged that are to a great extent independent of national boundaries. As a result, a new kind of war is being waged in every country all over the world; this is because the primary threats to national security have changed fundamentally. They no longer spring from territorial and ideological disputes among nation states but from how far globalization, technological threats and criminal networks have grown to challenge nation states (Zalur & Zeckhauser 2002).