Reprogramming of Isocitrate Dehydrogenases Expression and Activity by the Androgen
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download (Pdf)
Invivoscribe's wholly-owned Laboratories for Personalized Molecular LabPMM LLC Medicine® (LabPMM) is a network of international reference laboratories that provide the medical and pharmaceutical communities with worldwide Located in San Diego, California, USA, it holds access to harmonized and standardized clinical testing services. We view the following accreditations and certifications: internationally reproducible and concordant testing as a requirement for ISO 15189, CAP, and CLIA, and is licensed to provide diagnostic consistent stratification of patients for enrollment in clinical trials, and the laboratory services in the states of California, Florida, foundation for establishing optimized treatment schedules linked to patient’s Maryland, New York, Pennsylvania, and Rhode Island. individual profile. LabPMM provides reliable patient stratification at diagnosis LabPMM GmbH and monitoring, throughout the entire course of treatment in support of Personalized Molecular Medicine® and Personalized Based in Martinsried (Munich), Germany. It is an ISO 15189 Molecular Diagnostics®. accredited international reference laboratory. CLIA/CAP accreditation is planned. Invivoscribe currently operates four clinical laboratories to serve partners in the USA (San Diego, CA), Europe (Munich, Germany), and Asia (Tokyo, Japan and Shanghai, China). These laboratories use the same critical LabPMM 合同会社 reagents and software which are developed consistently with ISO Located in Kawasaki (Tokyo), Japan and a licensed clinical lab. 13485 design control. Our cGMP reagents, rigorous standards for assay development & validation, and testing performed consistently under ISO 15189 requirements help ensure LabPMM generates standardized and concordant test results worldwide. Invivoscribe Diagnostic Technologies (Shanghai) Co., Ltd. LabPMM is an international network of PersonalMed Laboratories® focused on molecular oncology biomarker studies. Located in Shangai, China. -

IDH2 Mutations—Co-Opting Cellular Metabolism for Malignant Transformation Eytan M
Published OnlineFirst November 9, 2015; DOI: 10.1158/1078-0432.CCR-15-0362 Molecular Pathways Clinical Cancer Research Molecular Pathways: IDH2 Mutations—Co-opting Cellular Metabolism for Malignant Transformation Eytan M. Stein Abstract Mutations in mitochondrial IDH2, one of the three isoforms function that catalyzes the conversion of alpha-ketoglutarate to of IDH, were discovered in patients with gliomas in 2009 and beta-hydroxyglutarate (2-HG). Supranormal levels of 2-HG subsequently described in acute myelogenous leukemia (AML), lead to hypermethylation of epigenetic targets and a subse- angioimmunoblastic T-cell lymphoma, chondrosarcoma, and quent block in cellular differentiation. AG-221, a small-mole- intrahepatic chloangiocarcinoma. The effects of mutations in cule inhibitor of mutant IDH2, is being explored in a phase I IDH2 on cellular metabolism, the epigenetic state of mutated clinical trial for the treatment of AML, other myeloid malig- cells, and cellular differentiation have been elucidated in vitro nancies, solid tumors, and gliomas. Clin Cancer Res; 22(1); 16–19. and in vivo.MutationsinIDH2 lead to an enzymatic gain of Ó2015 AACR. Background Interestingly, in a screen of 398 patients with AML, TET2 and IDH2 mutations were mutually exclusive, suggesting that these Isocitrate dehydrogenase (IDH) catalyzes the conversion of two mutations have a similar function. Wild-type TET2 demethy- isocitrate to alpha-ketoglutarate. IDH occurs in three isoforms, lates DNA, and mutations in TET2 lead to increases in 5-methyl- IDH1, located in the cytoplasm, IDH2 located in the mitochon- cytosine similar to those seen in patients with IDH mutations. It dria, and IDH3, which functions as part of the tricarboxylic acid has been suggested that elevated levels of 2-HG caused by mutant cycle. -

Anti-IDH3A Antibody (ARG42205)
Product datasheet [email protected] ARG42205 Package: 100 μl anti-IDH3A antibody Store at: -20°C Summary Product Description Rabbit Polyclonal antibody recognizes IDH3A Tested Reactivity Hu, Ms, Rat Tested Application ICC/IF, IHC-P, WB Host Rabbit Clonality Polyclonal Isotype IgG Target Name IDH3A Antigen Species Human Immunogen Recombinant fusion protein corresponding to aa. 28-366 of Human IDH3A (NP_005521.1). Conjugation Un-conjugated Alternate Names Isocitrate dehydrogenase [NAD] subunit alpha, mitochondrial; EC 1.1.1.41; NAD; Isocitric dehydrogenase subunit alpha; + Application Instructions Application table Application Dilution ICC/IF 1:50 - 1:200 IHC-P 1:50 - 1:200 WB 1:500 - 1:2000 Application Note * The dilutions indicate recommended starting dilutions and the optimal dilutions or concentrations should be determined by the scientist. Positive Control Raji Calculated Mw 40 kDa Observed Size ~ 40 kDa Properties Form Liquid Purification Affinity purified. Buffer PBS (pH 7.3), 0.02% Sodium azide and 50% Glycerol. Preservative 0.02% Sodium azide Stabilizer 50% Glycerol Storage instruction For continuous use, store undiluted antibody at 2-8°C for up to a week. For long-term storage, aliquot and store at -20°C. Storage in frost free freezers is not recommended. Avoid repeated freeze/thaw www.arigobio.com 1/3 cycles. Suggest spin the vial prior to opening. The antibody solution should be gently mixed before use. Note For laboratory research only, not for drug, diagnostic or other use. Bioinformation Gene Symbol IDH3A Gene Full Name isocitrate dehydrogenase 3 (NAD+) alpha Background Isocitrate dehydrogenases catalyze the oxidative decarboxylation of isocitrate to 2-oxoglutarate. -

Src-Family Kinases Impact Prognosis and Targeted Therapy in Flt3-ITD+ Acute Myeloid Leukemia
Src-Family Kinases Impact Prognosis and Targeted Therapy in Flt3-ITD+ Acute Myeloid Leukemia Title Page by Ravi K. Patel Bachelor of Science, University of Minnesota, 2013 Submitted to the Graduate Faculty of School of Medicine in partial fulfillment of the requirements for the degree of Doctor of Philosophy University of Pittsburgh 2019 Commi ttee Membership Pa UNIVERSITY OF PITTSBURGH SCHOOL OF MEDICINE Commi ttee Membership Page This dissertation was presented by Ravi K. Patel It was defended on May 31, 2019 and approved by Qiming (Jane) Wang, Associate Professor Pharmacology and Chemical Biology Vaughn S. Cooper, Professor of Microbiology and Molecular Genetics Adrian Lee, Professor of Pharmacology and Chemical Biology Laura Stabile, Research Associate Professor of Pharmacology and Chemical Biology Thomas E. Smithgall, Dissertation Director, Professor and Chair of Microbiology and Molecular Genetics ii Copyright © by Ravi K. Patel 2019 iii Abstract Src-Family Kinases Play an Important Role in Flt3-ITD Acute Myeloid Leukemia Prognosis and Drug Efficacy Ravi K. Patel, PhD University of Pittsburgh, 2019 Abstract Acute myelogenous leukemia (AML) is a disease characterized by undifferentiated bone-marrow progenitor cells dominating the bone marrow. Currently the five-year survival rate for AML patients is 27.4 percent. Meanwhile the standard of care for most AML patients has not changed for nearly 50 years. We now know that AML is a genetically heterogeneous disease and therefore it is unlikely that all AML patients will respond to therapy the same way. Upregulation of protein-tyrosine kinase signaling pathways is one common feature of some AML tumors, offering opportunities for targeted therapy. -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -
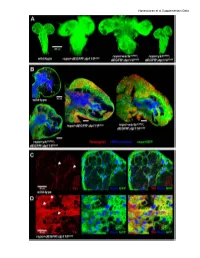
Supplementary Data Vigneswaran Et Al Supplementary Data
Vigneswaran et al Supplementary Data Vigneswaran et al Supplementary Data Figure S1: Yki is required for EGFR-PI3K-driven glial neoplasia in Drosophila (A) Optical projections of whole brain-nerve cord complexes from 3rd instar larvae approximately 130 hrs old. Dorsal view; anterior up. CD8-GFP (green) labels glial cell bodies. Compared to repo>dEGFRλ;dp110CAAX, warts knockdown (repo>wartsdsRNA; dEGFRλ;dp110CAAX) increased neoplastic brain overgrowth and yki knockdown (repo>ykidsRNA;dEGFRλ;dp110CAAX) decreased neoplastic brain overgrowth. (B) 3 µm optical projections of brain hemispheres, age-matched 3rd instar larvae. Frontal sections; anterior up; midline to left. Repo (red) labels glial cell nuclei; CD8-GFP (green) labels glial cell bodies; anti-HRP (blue) counter-stains for neurons and neuropil. (middle) repo>dEGFRλ;dp110CAAX showed increased glial cell numbers (red nuclei) compared to (upper left) wild-type. Compared to repo>dEGFRλ;dp110CAAX, (right) warts knockdown increased neoplastic glial cell numbers (red nuclei), whereas (lower left) yki knockdown reduced neoplastic glial cell numbers (red nuclei). (C, D) Low levels of Yki protein (red) was observed in wild-type central brain glia (white arrows, left panel in C) compared to high levels of cytoplasmic and nuclear Yki protein in dEGFRλ;dp110CAAX neoplastic glia (white arrows, left panel in D); Repo (blue) labels glial cell nuclei; CD8-GFP (green) labels glial cell bodies. Vigneswaran et al Supplementary Data Figure S2: YAP/TAZ expression confined to RTK-amplified tuMor cells and Maintained in patient-derived xenografts (A) On the left, immunohistochemical (IHC) staining in representative normal brain parenchyma in the cortex where YAP expression and TAZ expression was limited to vascular cells and was not detectable in normal neuronal and glial cells. -

Potential Genotoxicity from Integration Sites in CLAD Dogs Treated Successfully with Gammaretroviral Vector-Mediated Gene Therapy
Gene Therapy (2008) 15, 1067–1071 & 2008 Nature Publishing Group All rights reserved 0969-7128/08 $30.00 www.nature.com/gt SHORT COMMUNICATION Potential genotoxicity from integration sites in CLAD dogs treated successfully with gammaretroviral vector-mediated gene therapy M Hai1,3, RL Adler1,3, TR Bauer Jr1,3, LM Tuschong1, Y-C Gu1,XWu2 and DD Hickstein1 1Experimental Transplantation and Immunology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland, USA and 2Laboratory of Molecular Technology, Scientific Applications International Corporation-Frederick, National Cancer Institute-Frederick, Frederick, Maryland, USA Integration site analysis was performed on six dogs with in hematopoietic stem cells. Integrations clustered around canine leukocyte adhesion deficiency (CLAD) that survived common insertion sites more frequently than random. greater than 1 year after infusion of autologous CD34+ bone Despite potential genotoxicity from RIS, to date there has marrow cells transduced with a gammaretroviral vector been no progression to oligoclonal hematopoiesis and no expressing canine CD18. A total of 387 retroviral insertion evidence that vector integration sites influenced cell survival sites (RIS) were identified in the peripheral blood leukocytes or proliferation. Continued follow-up in disease-specific from the six dogs at 1 year postinfusion. A total of 129 RIS animal models such as CLAD will be required to provide an were identified in CD3+ T-lymphocytes and 102 RIS in accurate estimate -

As a Potential Therapeutic Approach for AML Wittawat Chantkran 1,2,Ya-Chinghsieh1, Daniella Zheleva3,Sheelaghframe3, Helen Wheadon1 and Mhairi Copland 1
Chantkran et al. Cell Death Discovery (2021) 7:137 https://doi.org/10.1038/s41420-021-00496-y Cell Death Discovery ARTICLE Open Access Interrogation of novel CDK2/9 inhibitor fadraciclib (CYC065) as a potential therapeutic approach for AML Wittawat Chantkran 1,2,Ya-ChingHsieh1, Daniella Zheleva3,SheelaghFrame3, Helen Wheadon1 and Mhairi Copland 1 Abstract Over the last 50 years, there has been a steady improvement in the treatment outcome of acute myeloid leukemia (AML). However, median survival in the elderly is still poor due to intolerance to intensive chemotherapy and higher numbers of patients with adverse cytogenetics. Fadraciclib (CYC065), a novel cyclin-dependent kinase (CDK) 2/9 inhibitor, has preclinical efficacy in AML. In AML cell lines, myeloid cell leukemia 1 (MCL-1) was downregulated following treatment with fadraciclib, resulting in a rapid induction of apoptosis. In addition, RNA polymerase II (RNAPII)-driven transcription was suppressed, rendering a global gene suppression. Rapid induction of apoptosis was observed in primary AML cells after treatment with fadraciclib for 6–8 h. Twenty-four hours continuous treatment further increased efficacy of fadraciclib. Although preliminary results showed that AML cell lines harboring KMT2A rearrangement (KMT2A-r) are more sensitive to fadraciclib, we found that the drug can induce apoptosis and decrease MCL-1 expression in primary AML cells, regardless of KMT2A status. Importantly, the diversity of genetic mutations observed in primary AML patient samples was associated with variable response to fadraciclib, confirming the need for fi 1234567890():,; 1234567890():,; 1234567890():,; 1234567890():,; patient strati cation to enable a more effective and personalized treatment approach. Synergistic activity was demonstrated when fadraciclib was combined with the BCL-2 inhibitor venetoclax, or the conventional chemotherapy agents, cytarabine, or azacitidine, with the combination of fadraciclib and azacitidine having the most favorable therapeutic window. -

Mouse Idh3a Mutations Cause Retinal Degeneration and Reduced Mitochondrial Function Amy S
© 2018. Published by The Company of Biologists Ltd | Disease Models & Mechanisms (2018) 11, dmm036426. doi:10.1242/dmm.036426 RESEARCH ARTICLE Mouse Idh3a mutations cause retinal degeneration and reduced mitochondrial function Amy S. Findlay1, Roderick N. Carter2, Becky Starbuck3, Lisa McKie1, Klára Nováková1, Peter S. Budd1, Margaret A. Keighren1, Joseph A. Marsh1, Sally H. Cross1, Michelle M. Simon3, Paul K. Potter3, Nicholas M. Morton2 and Ian J. Jackson1,4,* ABSTRACT these diseases have a wide range of pathologies (Koopman et al., Isocitrate dehydrogenase (IDH) is an enzyme required for the 2012). Common to many of the diseases, however, are neurological production of α-ketoglutarate from isocitrate. IDH3 generates the or neuromuscular manifestations, including retinal disease. We have NADH used in the mitochondria for ATP production, and is a tetramer identified a missense mutation in the gene encoding a subunit of the made up of two α, one β and one γ subunit. Loss-of-function and tricarboxylic acid (TCA) cycle enzyme isocitrate dehydrogenase 3 missense mutations in both IDH3A and IDH3B have previously been (IDH3) as causing a retinal degeneration phenotype in mice. There implicated in families exhibiting retinal degeneration. Using mouse are three isocitrate dehydrogenase isozymes in mammals. IDH1 and models, we investigated the role of IDH3 in retinal disease and IDH2 are both homodimers that catalyse the decarboxylation of α mitochondrial function. We identified mice with late-onset retinal isocitrate to -ketoglutarate with the concomitant reduction of NADP degeneration in a screen of ageing mice carrying an ENU-induced to NADPH. IDH1 is cytoplasmic, whereas IDH2 is localised to the mutation, E229K, in Idh3a. -

Supplementary Table 1 Genes Tested in Qrt-PCR in Nfpas
Supplementary Table 1 Genes tested in qRT-PCR in NFPAs Gene Bank accession Gene Description number ABI assay ID a disintegrin-like and metalloprotease with thrombospondin type 1 motif 7 ADAMTS7 NM_014272.3 Hs00276223_m1 Rho guanine nucleotide exchange factor (GEF) 3 ARHGEF3 NM_019555.1 Hs00219609_m1 BCL2-associated X protein BAX NM_004324 House design Bcl-2 binding component 3 BBC3 NM_014417.2 Hs00248075_m1 B-cell CLL/lymphoma 2 BCL2 NM_000633 House design Bone morphogenetic protein 7 BMP7 NM_001719.1 Hs00233476_m1 CCAAT/enhancer binding protein (C/EBP), alpha CEBPA NM_004364.2 Hs00269972_s1 coxsackie virus and adenovirus receptor CXADR NM_001338.3 Hs00154661_m1 Homo sapiens Dicer1, Dcr-1 homolog (Drosophila) (DICER1) DICER1 NM_177438.1 Hs00229023_m1 Homo sapiens dystonin DST NM_015548.2 Hs00156137_m1 fms-related tyrosine kinase 3 FLT3 NM_004119.1 Hs00174690_m1 glutamate receptor, ionotropic, N-methyl D-aspartate 1 GRIN1 NM_000832.4 Hs00609557_m1 high-mobility group box 1 HMGB1 NM_002128.3 Hs01923466_g1 heterogeneous nuclear ribonucleoprotein U HNRPU NM_004501.3 Hs00244919_m1 insulin-like growth factor binding protein 5 IGFBP5 NM_000599.2 Hs00181213_m1 latent transforming growth factor beta binding protein 4 LTBP4 NM_001042544.1 Hs00186025_m1 microtubule-associated protein 1 light chain 3 beta MAP1LC3B NM_022818.3 Hs00797944_s1 matrix metallopeptidase 17 MMP17 NM_016155.4 Hs01108847_m1 myosin VA MYO5A NM_000259.1 Hs00165309_m1 Homo sapiens nuclear factor (erythroid-derived 2)-like 1 NFE2L1 NM_003204.1 Hs00231457_m1 oxoglutarate (alpha-ketoglutarate) -

S6K1 Mediates Oncogenic Glycolysis in Pten Deficient Leukemia
S6K1 mediates oncogenic glycolysis in Pten deficient leukemia A dissertation submitted to the Graduate School of the University of Cincinnati in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Ph.D.) in the Department of Cancer and Cell Biology of the College of Medicine by Preeti Tandon M.S. Bowling Green State University, 2004 ABSTRACT Hyperactive Akt signaling triggers glycolysis and apoptosis resistance in human cancer. Because sustained glycolysis is required for Akt dependent apoptosis resistance, we investigated the downstream signaling components that mediate Akt dependent increases in glycolysis in cells deficient for Pten, a negative regulator of the PI3K/Akt pathway. Genetic inactivation of the ribosomal protein S6 Kinase 1 (S6K1) in Pten-deficient cells prevented glycolysis, triggered Bax translocation and committed cells to apoptosis. Pharmacological S6K1 inhibition using a small molecule kinase inhibitor recapitulated the effects of genetically inactivating S6K1. Inactivation of S6K1 was associated with decreased expression of the pro-glycolytic HIF1α transcription factor. Restoring HIF1α expression was sufficient to restore both glycolysis and cell survival in S6K1-deficient cells. Conversely, inhibiting HIF1α expression in Pten deficient cells resulted in decreased glycolysis and cell survival, mimicking the loss of S6K1. In vivo, S6K1 deficiency delayed the development of lethal disease in a Pten deficient mouse model of leukemia. Thus, together the data suggest that S6K1 is a useful target for counteracting the metabolic program that supports apoptosis resistance in Pten-deficient cancers. iii iv ACKNOWLEDGEMENTS I would first and foremost like to thank Dr David Plas for being my mentor. Your guidance and advice have been instrumental in my progress and development as a scientist. -

Targeting the Phosphatidylinositol 3-Kinase Signaling Pathway in Acute
Integrative Cancer Science and Therapeutics Review Article ISSN: 2056-4546 Targeting the phosphatidylinositol 3-kinase signaling pathway in acute myeloid leukemia Ota Fuchs* Institute of Hematology and Blood Transfusion, Prague, Czech Republic Abstract The phosphatidylinositol-3-kinase-Akt (protein kinase B) - mechanistic target of rapamycin (PI3K-Akt-mTOR) pathway is often dysregulated in cancer, including hematological malignancies. Primary acute myeloid leukemia (AML) cell populations may include various subclones at the time of diagnosis. A relapse can occur due to regrowth of the originally dominating clone, a subclone detectable at the time of first diagnosis, or a new clone derived either from the original clone or from remaining preleukemic stem cells. Inhibition of mTOR signaling has in general modest growth-inhibitory effects in preclinical AML models and clinical trials. Therefore, combination of allosteric mTOR inhibitors with standard chemotherapy or targeted agents has a greater anti-leukemia efficacy. Dual mTORC1/2 inhibitors, and dual PI3K/mTOR inhibitors show greater activity in pre-clinical AML models. Understanding the role of mTOR signaling in leukemia stem cells is important because AML stem cells may become chemoresistant by displaying aberrant signaling molecules, modifying epigenetic mechanisms, and altering the components of the bone marrow microenvironment. The PI3K/Akt/mTOR signaling pathway is promising target in the treatment of hematological malignancies, including AML, especially by using of combinations of mTOR inhibitors with conventional cytotoxic agents. Introduction syndromes, chronic myelogenous leukemia (CML), multiple myeloma and lymphoid leukemias and lymphomas [42-54]. Below, I discuss the The mammalian target of rapamycin (mTOR) is a serine/threonine PI3K/Akt/mTOR pathway and its role in AML.