Altrjra 1985 ENERGY and NATURAL RESOURCES Edmonton Fish and Wildlife Division ENR Technical Report Number: T/73 International Standard Book Number: 0-86499-985-2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Moose Lake Handbook
Moose Lake Our Past - Our Home - Our Future Moose Lake has a rich history starting with the fur trade and continually evolving to today with over 10,000 visitors annually. The area is under pressure from development such as subdivisions, lakelots, campgrounds, industry, agriculture and recreation; as well as the wildlife that make the lake their home. The Moose Lake Watershed Society (MLWS) recognizes that watershed management is vital to conserving the lake and maintaining its ecological value that we all can enjoy. The Moose Lake Water- shed Society is pleased to provide this handbook, which helps us achieve our vision as well as work to complete our Moose Lake Watershed Management Plan goals. Vision To maintain a healthy and func- tioning Moose Lake Watershed and recognize the importance of living within the capacity of the natural environment as a means of ensuring sustainable environ- mental, economic and social values. Acknowledgments This handbook was created for the Moose Lake Watershed Society by Kellie Nichiporik, with the assis- tance of the staff of the Beaver River Watershed Alliance and reviewed by members of the Moose Lake Watershed Society. Photos by Kellie Nichiporik. Thank you to the Lac La Nonne Enhancement and Protection Association, Waters Edge Resource Group and Lac La Nonne Watershed Stewardship Society for their pioneering accomplishments of the Lac La Nonne and Nakamun Lake Handbooks, upon which this project is based. Thank you to Bill Fox for providing the history of Moose Lake. Thank you to Lakeland Agricultural Research Association and Cows and Fish for providing resources and assistance. -
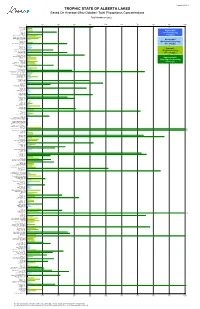
Trophic State of Alberta Lakes Based on Average Total Phosphorus
Created Feb 2013 TROPHIC STATE OF ALBERTA LAKES Based On Average (May-October) Total Phosphorus Concentrations Total Phosphorus (µg/L) 0 100 200 300 400 500 600 700 800 900 1000 * Adamson Lake Alix Lake * Amisk Lake * Angling Lake Oligotrophic * ‡ Antler Lake Arm Lake (Low Productivity) * Astotin Lake (<10 µg/L) * ‡ Athabasca (Lake) - Off Delta Baptiste Lake - North Basin Baptiste Lake - South Basin * ‡ Bare Creek Res. Mesotrophic * ‡ Barrier Lake ‡ Battle Lake (Moderate Productivity) * † Battle River Res. (Forestburg) (10 - 35 µg/L) Beartrap Lake Beauvais Lake Beaver Lake * Bellevue Lake Eutrophic * † Big Lake - East Basin * † Big Lake - West Basin (High Productivity) * Blackfalds Lake (35 - 100 µg/L) * † Blackmud Lake * ‡ Blood Indian Res. Bluet (South Garnier Lake) ‡ Bonnie Lake Hypereutrophic † Borden Lake * ‡ Bourque Lake (Very High Productivity) ‡ Buck Lake (>100 µg/L) Buffalo Lake - Main Basin Buffalo Lake - Secondary Bay * † Buffalo Lake (By Boyle) † Burntstick Lake Calling Lake * † Capt Eyre Lake † Cardinal Lake * ‡ Carolside Res. - Berry Creek Res. † Chain Lakes Res. - North Basin † Chain Lakes Res.- South Basin Chestermere Lake * † Chickakoo Lake * † Chickenhill Lake * Chin Coulee Res. * Clairmont Lake Clear (Barns) Lake Clear Lake ‡ Coal Lake * ‡ Cold Lake - English Bay ‡ Cold Lake - West Side ‡ Cooking Lake † Cow Lake * Crawling Valley Res. Crimson Lake Crowsnest Lake * † Cutbank Lake Dillberry Lake * Driedmeat Lake ‡ Eagle Lake ‡ Elbow Lake Elkwater Lake Ethel Lake * Fawcett Lake * † Fickle Lake * † Figure Eight Lake * Fishing Lake * Flyingshot Lake * Fork Lake * ‡ Fox Lake Res. Frog Lake † Garner Lake Garnier Lake (North) * George Lake * † Ghost Res. - Inside Bay * † Ghost Res. - Inside Breakwater ‡ Ghost Res. - Near Cochrane * Gleniffer Lake (Dickson Res.) * † Glenmore Res. -

Western Grebe Surveys in Alberta 2016
WESTERN GREBE SURVEYS IN ALBERTA 2016 The western grebe has been listed as a Threatened species in Alberta. A recent data compilation shows that there are approximately 250 lakes that have supported western grebes in Alberta. However, information for most lakes is poor and outdate d. Total counts on lakes are rare, breeding status is uncertain, and the location and extent of breeding habitat (emergent vegetation, usually bulrush) is usually unknown. We are seeking your help in gathering more information on western grebe populations in Alberta. If you visit any of the lakes listed below, or know anyone that does, we would appreciate as much detail as you can collect on the presence of western grebes and their habitat. Let us know in advance (if possible) if you are planning on going to any lakes, and when you do, e-mail details of your observations to [email protected]. SURVEY METHODS: Visit a lake between 1 May and 31 August with spotting scope or good binoculars. Surveys can be done from a boat, or vantage point(s) from shore. Report names of surveyors, dates, number of adults seen, and report on the approximate percentage of the lake area that this number represents. Record presence of young birds or nesting colonies, and provide any additional information on presence/location of likely breeding habitat, specific parts of the lake observed, observed threats to birds or habitat (boat traffic, shoreline clearing, pollution, etc.). Please report on findings even if no birds were seen. Lakes on the following page that are flagged with an asterisk (*) were not visited in 2015, and are priority for survey in 2016. -

Environmentally Significant Areas of Alberta Volume 2 Prepared By
Environmentally Significant Areas of Alberta Volume 2 Prepared by: Sweetgrass Consultants Ltd. Calgary, AB for: Resource Data Division Alberta Environmental Protection Edmonton, Alberta March 1997 EXECUTIVE SUMMARY Large portions of native habitats have been converted to other uses. Surface mining, oil and gas exploration, forestry, agricultural, industrial and urban developments will continue to put pressure on the native species and habitats. Clearing and fragmentation of natural habitats has been cited as a major area of concern with respect to management of natural systems. While there has been much attention to managing and protecting endangered species, a consensus is emerging that only a more broad-based ecosystem and landscape approach to preserving biological diversity will prevent species from becoming endangered in the first place. Environmentally Significant Areas (ESAs) are important, useful and often sensitive features of the landscape. As an integral component of sustainable development strategies, they provide long-term benefits to our society by maintaining ecological processes and by providing useful products. The identification and management of ESAs is a valuable addition to the traditional socio-economic factors which have largely determined land use planning in the past. The first ESA study done in Alberta was in 1983 for the Calgary Regional Planning Commission region. Numerous ESA studies were subsequently conducted through the late 1980s and early 1990s. ESA studies of the Parkland, Grassland, Canadian Shield, Foothills and Boreal Forest Natural Regions are now all completed while the Rocky Mountain Natural Region has been only partially completed. Four factors regarding the physical state of the site were considered when assessing the overall level of significance of each ESA: representativeness, diversity, naturalness, and ecological integrity. -

BONNYVILLE WATERSHED BEACON CORNER a Watershed/Drainage Basin Is the Water from an Area of Land Which Flows Into One Big Body of Water
480000 500000 520000 540000 560000 Harold Lake KEY MAP Manatokan 892 Lake VU COLD Cold Lake à TRUMAN Ä LAKE Meadow Lake M a n a t o k a n 55 149A Provincial Park à S u b - b a s i n Ä Twp.63 Rge.2 28 Ã Ä Rge.5 Rge.9 IRON RIVER Rge.6 LA COREY Edward Lake Rge.4 Rge.3 Rge.1 Rge.10 Rge.8 Rge.7 55 M a r i e C r e e k Cold Lake French Provincial Park W4M S a n d k A L B E R TA Bay PRA e S u b - b a s i n à J a c k f i s h Ä re R i v e r C n a C r e e k LESSARD 63 k o 897 t VU S u b - b a s i n a n a S u b - b a s i n à M Ä Jackfish Creek L o w e r 36 897 à B e a v e r R i v e r VU Ä S u b - b a s i n C o l d L a k e 55 COLD LAKE (! COLD LAKE S u b - b a s i n S A S K A T C H E W A N (! BONNYVILLE BIG MEADOW Ã Ã Ä Ä Ã 28 18 Ä GOODRIDGE (! 28 r SMOKY LAKE e v i S R (! 881 ! a d ( à VU Ä n iv e R e r ST. -

2001 Stocking Program
Fisheries Management Information System Report : Stocking Report Module Id : FM_RRSTK Filename : H:fm_rrstk.pdf Run by : JWAGNER Report Date: 02-NOV-2001 For Year: 2001 Stocking Report for year: 2001 Page 2 of 8 Sport Fishing Zone: ES1 Oldman / Bow River Watershed Location Month Number Species Ave. Length (cm) AIRDRIE POND (1-27-1-W5) May 250 RNTR 21 AIRDRIE POND (1-27-1-W5) June 250 RNTR 20 ALLEN BILL POND (30-22-5-W5) May 2,900 RNTR 23 ALLEN BILL POND (30-22-5-W5) June 2,900 RNTR 22 ALLISON LAKE (27-8-5-W5) May 3,800 RNTR 28 BATHING LAKE (11-4-1-W5) May 710 RNTR 19 BEAR POND (36-14-4-W5) June 1,800 ARGR BEAUVAIS LAKE (29-5-1-W5) May 46,000 RNTR 17 BEAUVAIS LAKE (29-5-1-W5) June 580 BNTR 23 BEAUVAIS LAKE (29-5-1-W5) September 58,700 BNTR 10 BEAVER MINES LAKE (11-5-3-W5) May 65,100 RNTR 9 BEAVER MINES LAKE (11-5-3-W5) May 19,300 RNTR 10 BEAVER MINES LAKE (11-5-3-W5) August 10,800 RNTR 10 BIG IRON LAKE (1-15-4-W5) June 2,500 ARGR BULLER POND (20-22-10-W5) June 1,300 RNTR 22 BURMIS LAKE (14-7-3-W5) May 1,000 RNTR 19 BURN'S RESERVOIR (26-6-30-W4) April 500 RNTR 21 BUTCHER'S LAKE (15-4-1-W5) August 6,400 BKTR 11 CHAIN LAKES RESERVOIR (4-15-2-W5) May 121,500 RNTR 12 CHAIN LAKES RESERVOIR (4-15-2-W5) May 55,700 RNTR 13 CHAIN LAKES RESERVOIR (4-15-2-W5) May 14,500 RNTR 14 CHAIN LAKES RESERVOIR (4-15-2-W5) June 14,400 RNTR 14 CHAIN LAKES RESERVOIR (4-15-2-W5) July 8,100 RNTR 18 COLEMAN FISH AND GAME POND (24-8-5-W5) May 1,600 RNTR 19 COTTONWOOD LAKE (16-7-29-W4) May 750 RNTR 19 CROWSNEST LAKE (8-8-5-W5) January 500 RNTR 64 CROWSNEST LAKE (8-8-5-W5) -

" Wisconsin Lakes" PUB-FH-800 2005Rev
Wisconsin Lakes Wisconsin Department of Natural Resources PUB-FH-800 2009 Wisconsin Lakes This publication produced by Bureau of Fisheries and Habitat Management This publication funded in part by the Aquatic Resources Trust Fund—Sport Fish Restoration Program © Copyright 2009, Wisconsin Department of Natural Resources The Wisconsin Department of Natural Resources provides equal opportunity in its employment, programs, services and functions under an Affirmative Action Plan. If you have any questions, please write to Equal Opportunity Office, Department of the Interior, Washington, D.C. 20240. This publication is available in alternate format (large print, Braille, audio tape, etc.) upon request. Call 608-267-7498 for more information. Printed on Recycled Paper Preface This booklet is a public reference for Wisconsin lakes. It provides information on the physical attributes of lakes such as the size, depth, type of public access, availability of lake maps, the relative abundance of fish species, exotic plants and animals and information about fish consumption advisories. Observations and data collected in the 1950s and 1960s by Department of Natural Resources field staff form the base of information for this publication. Continual field work and input from staff and the public over the years provide updates on changing conditions of some, but not all lakes listed herein. This publication will be updated periodically, to reflect the most current informa- tion available on Wisconsin’s lakes. Please notify the DNR field station nearest you (refer to the back cover) if you are aware of any omissions, errors, or changes that require attention in the next edition of Wisconsin Lakes. -

By HFNICHOLSON Great Lakes Biolimnol
BIBLIOGRAPHY -ON THE LIMNOLOGY AND FISHERIES. OF CANADIAN FRESHWATERS. NO .''5 (REVISED). by H.F.NICHOLSON Great Lakes Biolimnology Laboratory, Canada Centre for Inland Waters, 867 Lakeshore Road, Burlington, Ontario. L7R 4A6 PREFACE This is a revised edition of Bibliography No.5, published in 1978 as Fish. Environm.Can., Fish.Mar.Serv., Techn.Rept., (804). Due to budget restrictions and the high cost of printing, combined with an expanding distribution list, it is no longer possible to publish this series as Technical Reports. Instead, each number will be issued in this present looseleaf form as an unpublished report of the Great Lakes Biolimnology Laboratory. Please note that those from outside Canada requesting copies of this series will be sent the Reference Indexes only. However, the Canadian Freshwater Features Section will be sent if specifically requested. This issue can be referenced as:- Nicholson, H.F. 1982. "Bibliography on the limnology and fisheries of Canadian freshwaters. No.5(revised)". Can.Dept.Fish.Oceans, Pacific & Freshw.Fish., Great Lakes Biolimnol.Lab., Unpubl.Rept. FORMAT The bibliography is divided into two sections:- (1). Reference Index Each of these references contains information on the limnology and fisheries of Canadian freshwaters. They are numbered and appear in numerical order. This enumeration is consecutive and continuous through the bibliography series. (2). Freshwater Feature Index This section is divided into alphabetical order of provinces and within each province the freshwater feature names are in alphabetical order. The coordinates (in minutes and degrees, latitude and longitude) are given for each feature except for British Columbia where, for the most part, the quadrilateral indexing system is used. -

2007 Stocking Program
Fisheries Management Information System Report : Stocking Report Module Id : FM_RRSTK Filename : H:fm_rrstk.pdf Run by : JWAGNER Report Date: 20-JUL-2007 For Year: 2007 Stocking Report for year: 2007 Page 2 of 8 Sport Fishing Zone: ES1 Oldman / Bow River Watershed Location Month Number Species Ave. Length (cm) AIRDRIE POND (1-27-1-W5) April 250 RNTR 21 AIRDRIE POND (1-27-1-W5) June 250 RNTR 20 ALLISON LAKE (27-8-5-W5) May 3,700 RNTR 21 ALLISON LAKE (27-8-5-W5) June 700 RNTR 25 BATHING LAKE (11-4-1-W5) May 750 RNTR 22 BEAUVAIS LAKE (29-5-1-W5) May 890 BNTR 19 BEAUVAIS LAKE (29-5-1-W5) May 4,900 RNTR 17 BEAUVAIS LAKE (29-5-1-W5) May 18,500 RNTR 18 BEAUVAIS LAKE (29-5-1-W5) June 25,100 BNTR 8 BEAVER MINES LAKE (11-5-3-W5) March 46 RNTR 51 BEAVER MINES LAKE (11-5-3-W5) June 36,900 RNTR 8 BULLER POND (20-22-10-W5) May 1,300 RNTR 27 BURMIS LAKE (14-7-3-W5) April 1,000 RNTR 21 BURN'S RESERVOIR (26-6-30-W4) April 500 RNTR 21 BURN'S RESERVOIR (26-6-30-W4) May 600 RNTR 22 CHAIN LAKES RESERVOIR (4-15-2-W5) March 80 RNTR 69 CHAIN LAKES RESERVOIR (4-15-2-W5) May 98,800 RNTR 14 CHAIN LAKES RESERVOIR (4-15-2-W5) May 50,300 RNTR 15 CHAIN LAKES RESERVOIR (4-15-2-W5) June 3,200 BLTR 22 CHAIN LAKES RESERVOIR (4-15-2-W5) June 9,700 RNTR 12 CHAIN LAKES RESERVOIR (4-15-2-W5) June 29,300 RNTR 14 CHAIN LAKES RESERVOIR (4-15-2-W5) June 17,000 RNTR 15 COLEMAN FISH AND GAME POND (24-8-5-W5) May 1,600 RNTR 22 COTTONWOOD LAKE (16-7-29-W4) April 750 RNTR 21 CROSSFIELD TROUT POND (27-28-1-W5) May 1,400 RNTR 26 CROWSNEST LAKE (8-8-5-W5) June 30,400 RNTR 10 CROWSNEST -

Jessie Lake Environmental Overview FINAL Secured
FINAL Environmental Overview Proposed Range Rd. 460 Improvements Municipal District of Bonnyville, Alberta NE 1-64-6-W4M Project 2014.08 Revised August 2014 Prepared For: SE Design and Consulting Inc. PO Box 151 Cold Lake, AB T9M 1P4 18931-111 Avenue NW, Edmonton, Alberta, T5S 2X4 Phone (780) 455-4292, www.green-plan.com, E Mail: [email protected] Environmental Overview – Jessie Lake Rd. October, 2014 Executive Summary Impact Assessment The re-surfacing road work will be undertaken in two phases; Phase I: the existing recycle asphalt will be removed and hauled off-site; Phase II: the base will be re-worked and a new surface applied. No road widening will take place and therefore works will not occur outside of the existing right-of-way footprint. Accordingly, the overall environmental risk associated with the project is considered low. The main concerns relate to sediment control and asphalt and oil migration into Jessie Lake and minor annoyances to the public related to traffic and pedestrian detours. Recommended mitigation measures to minimize potential impact are provided below. Recommendations General: • The quality of the aquatic environment adjacent to the site after construction, at a minimum should be equivalent to or exceed that which existed prior. • Isolate the work from the waterbody with a silt fence. • All equipment entering the project area should be maintained for fluid leaks. • All equipment entering the site should be clean of debris; invasive species and noxious weeds to eliminate the potential for bioinvasives. Erosion and Sediment Control: • Install erosion and sediment control measures before conducting earthworks. -

Bibliography on the Limnology and Fisheries of Canadian Freshwaters
BIBLIOGRAPHY ON THE LIMNOLOGY AND FISHERIES OF CANADIAN FRESHWATERS. N0.2(REVISED). by H.F.NICHOLSON Great Lakes Biolimnology Laboratory, Canada Centre for Inland Waters, 867 Lakeshore Road, Burlington, Ontario. L7R 4A6 1982 PREFACE This is a revised edition of Bibliography No.2, published in 1975 as Environm. Can., Fish.Mar.Serv., Techn.Rept., (504). Due to budget restrictions and the high cost of printing, combined with an expanding distribution list, it is no longer possible to publish this series as Technical Reports. Instead, each number will be issued in this present looseleaf form as an unpublished report of the Great Lakes Biolimnology Laboratory. Please note that those from outside Canada requesting copies of this series will be sent the Reference Indexes only, unless otherwise requested. This issue can be referenced as:- Nicholson, H.F. 1982. "Bibliography on the limnology and fisheries of Canadian freshwaters. No.2(revised)". Can.Dept.Fish.Oceans, Pacific & Freshw.Fish., Great Lakes Biolimnol.Lab., Unpubl.Rept. FORMAT The bibliography is divided into two sections:- (1). Reference Index Each of these references contains information on the limnology and fisheries of Canadian freshwaters. They are numbered and appear in num~rical order. This enumeration is consecutive and continuous through the bibliography series. (2). Freshwater Feature Index This section is divided into alphabetical order of provinces and within each province the freshwater feature names are in alphabetical order. The coordinates (in minutes and degrees, latitude and longitude) are given for each feature except for British Columbia where, for the most part, the quadrilateral indexing system is used. Apart from French names, it is usual for the specific name to precede the generic name, as in Elliot Lake, but in a few cases the reverse is true, such as Lake Nipissing, in which case the latter will appear as Nipissing, Lake, with a comma after the-specific name. -

NSWA Technical Bulletin Lake Level Trends in Alberta ‐ Preliminary Results
August 2017 www.nswa.ab.ca NSWA Technical Bulletin Lake Level Trends in Alberta ‐ Preliminary Results Chickakoo Lake – Bill Trout Introduction extremes). The Prairie provinces are particularly Canada is a land of lakes. It has more than a vulnerable to climate change effects, and some million lakes that cover 7.6% of the country’s area studies have described an overall pattern of th and is estimated to have approximately 20% of declining lake levels through most of the 20 4 the world’s existing freshwater reserves1. Lakes century . Some potential consequences of lake are an important source of water for a range of level fluctuations related to natural and human users (e.g. municipalities, agriculture, industry, stressors have been noticed in this region, for etc.), and are important ecosystems providing a example, water quality declines, increases in blue‐ multitude of ecological functions and a range of green algal warnings and fish kills. goods and recreational services. The North Saskatchewan Watershed Alliance Many studies in Canada have provided evidence (NSWA) encourages sustainable residential and of reduced water availability due to changing development practices in lake communities climate2,3. Lakes are not exempt from such within North Saskatchewan region. In this effects, and they are becoming more vulnerable context, the NSWA has been working with local to a range of stressors, both human (i.e. stewardship groups and municipalities to develop increasing consumptive use of water linked to lake management plans to protect and improve increased population and economic growth), and water quality and the health of the lakes. The natural (i.e.