Enrico Fermi's Impact on Science
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

James Chadwick: Ahead of His Time
July 15, 2020 James Chadwick: ahead of his time Gerhard Ecker University of Vienna, Faculty of Physics Boltzmanngasse 5, A-1090 Wien, Austria Abstract James Chadwick is known for his discovery of the neutron. Many of his earlier findings and ideas in the context of weak and strong nuclear forces are much less known. This biographical sketch attempts to highlight the achievements of a scientist who paved the way for contemporary subatomic physics. arXiv:2007.06926v1 [physics.hist-ph] 14 Jul 2020 1 Early years James Chadwick was born on Oct. 20, 1891 in Bollington, Cheshire in the northwest of England, as the eldest son of John Joseph Chadwick and his wife Anne Mary. His father was a cotton spinner while his mother worked as a domestic servant. In 1895 the parents left Bollington to seek a better life in Manchester. James was left behind in the care of his grandparents, a parallel with his famous predecessor Isaac Newton who also grew up with his grandmother. It might be an interesting topic for sociologists of science to find out whether there is a correlation between children educated by their grandmothers and future scientific geniuses. James attended Bollington Cross School. He was very attached to his grandmother, much less to his parents. Nevertheless, he joined his parents in Manchester around 1902 but found it difficult to adjust to the new environment. The family felt they could not afford to send James to Manchester Grammar School although he had been offered a scholarship. Instead, he attended the less prestigious Central Grammar School where the teaching was actually very good, as Chadwick later emphasised. -

Luis Alvarez: the Ideas Man
CERN Courier March 2012 Commemoration Luis Alvarez: the ideas man The years from the early 1950s to the late 1980s came alive again during a symposium to commemorate the birth of one of the great scientists and inventors of the 20th century. Luis Alvarez – one of the greatest experimental physicists of the 20th century – combined the interests of a scientist, an inventor, a detective and an explorer. He left his mark on areas that ranged from radar through to cosmic rays, nuclear physics, particle accel- erators, detectors and large-scale data analysis, as well as particles and astrophysics. On 19 November, some 200 people gathered at Berkeley to commemorate the 100th anniversary of his birth. Alumni of the Alvarez group – among them physicists, engineers, programmers and bubble-chamber film scanners – were joined by his collaborators, family, present-day students and admirers, as well as scientists whose professional lineage traces back to him. Hosted by the Lawrence Berkeley National Laboratory (LBNL) and the University of California at Berkeley, the symposium reviewed his long career and lasting legacy. A recurring theme of the symposium was, as one speaker put it, a “Shakespeare-type dilemma”: how could one person have accom- plished all of that in one lifetime? Beyond his own initiatives, Alvarez created a culture around him that inspired others to, as George Smoot put it, “think big,” as well as to “think broadly and then deep” and to take risks. Combined with Alvarez’s strong scientific standards and great care in execut- ing them, these principles led directly to the awarding of two Nobel Luis Alvarez celebrating the announcement of his 1968 Nobel prizes in physics to scientists at Berkeley – George Smoot in 2006 prize. -
![I. I. Rabi Papers [Finding Aid]. Library of Congress. [PDF Rendered Tue Apr](https://docslib.b-cdn.net/cover/8589/i-i-rabi-papers-finding-aid-library-of-congress-pdf-rendered-tue-apr-428589.webp)
I. I. Rabi Papers [Finding Aid]. Library of Congress. [PDF Rendered Tue Apr
I. I. Rabi Papers A Finding Aid to the Collection in the Library of Congress Manuscript Division, Library of Congress Washington, D.C. 1992 Revised 2010 March Contact information: http://hdl.loc.gov/loc.mss/mss.contact Additional search options available at: http://hdl.loc.gov/loc.mss/eadmss.ms998009 LC Online Catalog record: http://lccn.loc.gov/mm89076467 Prepared by Joseph Sullivan with the assistance of Kathleen A. Kelly and John R. Monagle Collection Summary Title: I. I. Rabi Papers Span Dates: 1899-1989 Bulk Dates: (bulk 1945-1968) ID No.: MSS76467 Creator: Rabi, I. I. (Isador Isaac), 1898- Extent: 41,500 items ; 105 cartons plus 1 oversize plus 4 classified ; 42 linear feet Language: Collection material in English Location: Manuscript Division, Library of Congress, Washington, D.C. Summary: Physicist and educator. The collection documents Rabi's research in physics, particularly in the fields of radar and nuclear energy, leading to the development of lasers, atomic clocks, and magnetic resonance imaging (MRI) and to his 1944 Nobel Prize in physics; his work as a consultant to the atomic bomb project at Los Alamos Scientific Laboratory and as an advisor on science policy to the United States government, the United Nations, and the North Atlantic Treaty Organization during and after World War II; and his studies, research, and professorships in physics chiefly at Columbia University and also at Massachusetts Institute of Technology. Selected Search Terms The following terms have been used to index the description of this collection in the Library's online catalog. They are grouped by name of person or organization, by subject or location, and by occupation and listed alphabetically therein. -

Chem 103, Section F0F Unit I
Lecture 4 - Observations that Led to the Chem 103, Section F0F Nuclear Model of the Atom Unit I - An Overview of Chemistry Dalton’s theory proposed that atoms were indivisible particles. Lecture 4 • By the late 19th century, this aspect of Dalton’s theory was being challenged. • Work with electricity lead to the discovery of the electron, • Some observations that led to the nuclear model as a particle that carried a negative charge. for the structure of the atom • The modern view of the atomic structure and the elements • Arranging the elements into a (periodic) table 2 Lecture 4 - Observations that Led to the Lecture 4 - Observations that Led to the Nuclear Model of the Atom Nuclear Model of the Atom The cathode ray In 1897, J.J. Thomson (1856-1940) studies how cathode rays • Cathode rays were shown to be electrons are affected by electric and magnetic fields • This allowed him to determine the mass/charge ration of an electron Cathode rays are released by metals at the cathode 3 4 Lecture 4 - Observations that Led to the Lecture 4 - Observations that Led to the Nuclear Model of the Atom Nuclear Model of the Atom In 1897, J.J. Thomson (1856-1940) studies how cathode rays In 1897, J.J. Thomson (1856-1940) studies how cathode rays are affected by electric and magnetic fields are affected by electric and magnetic fields • Thomson estimated that the mass of an electron was less • Thomson received the 1906 Nobel Prize in Physics for his that 1/1000 the mass of the lightest atom, hydrogen!! work. -

Enrico Fermi
Fermi, Enrico Inventors and Inventions Enrico Fermi Italian American physicist Fermi helped develop Fermi-Dirac statistics, which liceo (secondary school) and, on the advice of Amidei, elucidate the group behavior of elementary particles. joined the Scuola Normale Superiore at Pisa. This elite He also developed the theory of beta decay and college, attached to the University of Pisa, admitted only discovered neutron-induced artificial radioactivity. forty of Italy’s top students, who were given free board Finally, he succeeded in producing the first sustained and lodging. Fermi performed exceedingly well in the nuclear chain reaction, which led to the discovery highly competitive entrance exam. He completed his of nuclear energy and the development of the university education after only four years of research and atomic bomb. studies, receiving his Ph.D. in physics from the Univer- sity of Pisa and his undergraduate diploma from the Born: September 29, 1901; Rome, Italy Scuola Normale Superiore in July, 1922. He became Died: November 28, 1954; Chicago, Illinois an expert theoretical physicist and a talented exper- Primary field: Physics imentalist. This rare combination provided a solid foun- Primary inventions: Controlled nuclear chain dation for all his subsequent inventions. reaction; Fermi-Dirac statistics; theory of beta decay Life’s Work After postdoctoral work at the University of Göttingen, Early Life in Germany (1922-1923), and the University of Leiden, Enrico Fermi (ehn-REE-koh FUR-mee) was the third in the Netherlands (fall, 1924), Fermi took an interim po- child of Alberto Fermi and Ida de Gattis. Enrico was very sition at the University of Florence in December, 1924. -

Appendix E Nobel Prizes in Nuclear Science
Nuclear Science—A Guide to the Nuclear Science Wall Chart ©2018 Contemporary Physics Education Project (CPEP) Appendix E Nobel Prizes in Nuclear Science Many Nobel Prizes have been awarded for nuclear research and instrumentation. The field has spun off: particle physics, nuclear astrophysics, nuclear power reactors, nuclear medicine, and nuclear weapons. Understanding how the nucleus works and applying that knowledge to technology has been one of the most significant accomplishments of twentieth century scientific research. Each prize was awarded for physics unless otherwise noted. Name(s) Discovery Year Henri Becquerel, Pierre Discovered spontaneous radioactivity 1903 Curie, and Marie Curie Ernest Rutherford Work on the disintegration of the elements and 1908 chemistry of radioactive elements (chem) Marie Curie Discovery of radium and polonium 1911 (chem) Frederick Soddy Work on chemistry of radioactive substances 1921 including the origin and nature of radioactive (chem) isotopes Francis Aston Discovery of isotopes in many non-radioactive 1922 elements, also enunciated the whole-number rule of (chem) atomic masses Charles Wilson Development of the cloud chamber for detecting 1927 charged particles Harold Urey Discovery of heavy hydrogen (deuterium) 1934 (chem) Frederic Joliot and Synthesis of several new radioactive elements 1935 Irene Joliot-Curie (chem) James Chadwick Discovery of the neutron 1935 Carl David Anderson Discovery of the positron 1936 Enrico Fermi New radioactive elements produced by neutron 1938 irradiation Ernest Lawrence -

EUGENE PAUL WIGNER November 17, 1902–January 1, 1995
NATIONAL ACADEMY OF SCIENCES E U G ENE PAUL WI G NER 1902—1995 A Biographical Memoir by FR E D E R I C K S E I T Z , E RICH V OG T , A N D AL V I N M. W E I NBER G Any opinions expressed in this memoir are those of the author(s) and do not necessarily reflect the views of the National Academy of Sciences. Biographical Memoir COPYRIGHT 1998 NATIONAL ACADEMIES PRESS WASHINGTON D.C. Courtesy of Atoms for Peace Awards, Inc. EUGENE PAUL WIGNER November 17, 1902–January 1, 1995 BY FREDERICK SEITZ, ERICH VOGT, AND ALVIN M. WEINBERG UGENE WIGNER WAS A towering leader of modern physics Efor more than half of the twentieth century. While his greatest renown was associated with the introduction of sym- metry theory to quantum physics and chemistry, for which he was awarded the Nobel Prize in physics for 1963, his scientific work encompassed an astonishing breadth of sci- ence, perhaps unparalleled during his time. In preparing this memoir, we have the impression we are attempting to record the monumental achievements of half a dozen scientists. There is the Wigner who demonstrated that symmetry principles are of great importance in quan- tum mechanics; who pioneered the application of quantum mechanics in the fields of chemical kinetics and the theory of solids; who was the first nuclear engineer; who formu- lated many of the most basic ideas in nuclear physics and nuclear chemistry; who was the prophet of quantum chaos; who served as a mathematician and philosopher of science; and the Wigner who was the supervisor and mentor of more than forty Ph.D. -

52 Annual Greenville County
nd 52 Annual Greenville County and South Carolina Regional 1A Science and Engineering Fair 2006 Sponsored by Rotary Club of Greenville Roper Mountain Science Center and The South Carolina Academy of Science th th st Judging- March 14 , Public Exhibition – March 15 , Award Ceremony - March 21 Palmetto EXPO Center – Greenville, South Carolina For Immediate Release March 7, 2006 Regional Science & Engineering Fair announces new Charles H. Townes Student Research Award GREENVILLE, SC – The Greenville County and South Carolina Regional Science and Engineering Fair announces a new award recognizing a local high school student for excellence in science research. The Charles H. Townes Student Research Award is the top award in the 52nd Annual Greenville County and South Carolina Regional Science & Engineering Fair to be held at the Palmetto Expo Center. The award honors the career of Greenville’s Charles H. Townes, who received the 1964 Nobel prize in Physics for his research leading to the invention of the laser. To encourage our local future scientists Dr. Townes wrote: “Being a scientist can be a wonderful career. Science involves exploration, figuring things out, understanding our universe and ourselves, and new discoveries. We live in an amazing universe, understanding it is fascinating and inspiring, and also leads to new possibilities for humans. Applied science or engineering uses our scientific understanding to provide new tools, solutions to problems, and remarkable improvements in human life. To do good science, it's helpful to learn all one can, be familiar with a variety of fields, and enjoy exploring. The more we know, the better we can fit ideas together to make new ones. -

Enrico Fermi: Genius
ANNIVERSARY Enrico Fermi: genius This year marks the centenary of the birth of Enrico Fermi, one of the giants of 20th- • century science, and one of the last physicists to be both an accomplished experimentalist and an influential theorist. Here, Gianni Battimelli of the University of Rome "La Sapienza" traces the life of a genius. Enrico Fermi was born on 29 September 1901 in Rome to a family with no scientific traditions. His passion for natural sciences, and in particular for physics, was stimulated and guided in his school years by an engineer and family friend, Adolph Amidei, who recognized Fermi's exceptional intellectual abilities and suggested admission to Pisa's Scuola Normale Superiore. After finishing high-school studies in Rome, in 1918 Fermi progressed to the prestigious Pisa Institute, after producing for the admission exam an essay on the characteristics of the propagation of sound, the authenticity of which the commissioners initially refused to believe. Studies at Pisa did not pose any particular difficulties for the young Fermi, despite his having to be largely self-taught using mate rial in foreign languages because nothing existed at the time in Fermi's group discovered the Italian on the new physics emerging around relativity and quantum radioactivity induced by theory. In those years in Italy, these new theories were absent from university teaching, and only mathematicians likeTullio Levi-Civita neutrons, instead of the had the knowledge and insight to see their implications. alpha particles used in the Working alone, between 1919 and 1922, Fermi built up a solid competence in relativity, statistical mechanics and the applications Paris experiments. -

Historical Background Prepared by Dr, Robin Chaplin Professor of Power Plant Engineering (Retired) University of New Brunswick
1 Historical Background prepared by Dr, Robin Chaplin Professor of Power Plant Engineering (retired) University of New Brunswick Summary: A review of the historical background for the development of nuclear energy is given to set the scene for the discussion of CANDU reactors. Table of Contents 1 Growth of Science and Technology......................................................................................... 2 2 Renowned Scientists............................................................................................................... 4 3 Significant Achievements........................................................................................................ 6 3.1 Niels Bohr........................................................................................................................ 6 3.2 James Chadwick .............................................................................................................. 6 3.3 Enrico Fermi .................................................................................................................... 6 4 Nuclear Fission........................................................................................................................ 7 5 Nuclear Energy........................................................................................................................ 7 6 Acknowledgments................................................................................................................... 8 List of Figures Figure 1 Timeline of significant discoveries -

Absolute Zero, Absolute Temperature. Absolute Zero Is the Lowest
Contents Radioactivity: The First Puzzles................................................ 1 The “Uranic Rays” of Henri Becquerel .......................................... 1 The Discovery ............................................................... 2 Is It Really Phosphorescence? .............................................. 4 What Is the Nature of the Radiation?....................................... 5 A Limited Impact on Scientists and the Public ............................ 6 Why 1896? .................................................................. 7 Was Radioactivity Discovered by Chance? ................................ 7 Polonium and Radium............................................................. 9 Marya Skłodowska .......................................................... 9 Pierre Curie .................................................................. 10 Polonium and Radium: Pierre and Marie Curie Invent Radiochemistry.. 11 Enigmas...................................................................... 14 Emanation from Thorium ......................................................... 17 Ernest Rutherford ........................................................... 17 Rutherford Studies Radioactivity: ˛-and ˇ-Rays.......................... 18 ˇ-Rays Are Electrons ....................................................... 19 Rutherford in Montreal: The Radiation of Thorium, the Exponential Decrease........................................... 19 “Induced” and “Excited” Radioactivity .................................... 20 Elster -
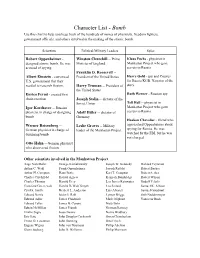
Character List
Character List - Bomb Use this chart to help you keep track of the hundreds of names of physicists, freedom fighters, government officials, and others involved in the making of the atomic bomb. Scientists Political/Military Leaders Spies Robert Oppenheimer - Winston Churchill -- Prime Klaus Fuchs - physicist in designed atomic bomb. He was Minister of England Manhattan Project who gave accused of spying. secrets to Russia Franklin D. Roosevelt -- Albert Einstein - convinced President of the United States Harry Gold - spy and Courier U.S. government that they for Russia KGB. Narrator of the needed to research fission. Harry Truman -- President of story the United States Enrico Fermi - created first Ruth Werner - Russian spy chain reaction Joseph Stalin -- dictator of the Tell Hall -- physicist in Soviet Union Igor Korchatov -- Russian Manhattan Project who gave physicist in charge of designing Adolf Hitler -- dictator of secrets to Russia bomb Germany Haakon Chevalier - friend who Werner Reisenberg -- Leslie Groves -- Military approached Oppenheimer about German physicist in charge of leader of the Manhattan Project spying for Russia. He was designing bomb watched by the FBI, but he was not charged. Otto Hahn -- German physicist who discovered fission Other scientists involved in the Manhattan Project: Aage Niels Bohr George Kistiakowsky Joseph W. Kennedy Richard Feynman Arthur C. Wahl Frank Oppenheimer Joseph Rotblat Robert Bacher Arthur H. Compton Hans Bethe Karl T. Compton Robert Serber Charles Critchfield Harold Agnew Kenneth Bainbridge Robert Wilson Charles Thomas Harold Urey Leo James Rainwater Rudolf Pelerls Crawford Greenewalt Harold DeWolf Smyth Leo Szilard Samuel K. Allison Cyril S. Smith Herbert L. Anderson Luis Alvarez Samuel Goudsmit Edward Norris Isidor I.