CVS CAREMARK CORPORATION (Exact Name of Registrant As Specified in Its Charter)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
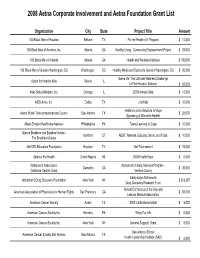
2008 Aetna Corporate Involvement and Aetna Foundation Grant List
2008 Aetna Corporate Involvement and Aetna Foundation Grant List Organization City State Project Title Amount 100 Black Men of Houston Bellaire TX For the Health of it Program$ 10,000 100 Black Men of America, Inc. Atlanta GA Healthy Living - Community Empowerment Project$ 25,000 100 Black Men of Atlanta Atlanta GA Health and Wellness Initiative$ 100,000 100 Black Men of Greater Washington, DC Washington DC Healthy Minds and Bodies for Greater Washington, DC$ 25,000 Game On! The Ultimate Wellness Challenge Action for Healthy Kids Skokie IL in Five Houston Schools $ 30,000 After-School Matters, Inc. Chicago IL 2008 Annual Gala$ 10,000 AIDS Arms, Inc. Dallas TX LifeWalk$ 10,000 Hablando de la Salud de la Mujer Alamo Public Telecommunications Council San Antonio TX $ 20,000 (Speaking of Women's Health) Albert Einstein Healthcare Network Philadelphia PA Teens Learning to Cope$ 10,000 Alianca Brasileira nos Estados Unidos - Hartford CT NEST: Network, Educate, Serve, and Track$ 10,000 The Brazilian Alliance Alief ISD Education Foundation Houston TX Golf Tournament$ 15,000 Alliance For Health Grand Rapids MI 2008 Health Expo$ 6,000 Alzheimer's Association, Alzheimer's Family Services Program - Camarillo CA $ 35,000 California Central Coast Ventura County Early-stage Alzheimer's Alzheimer's Drug Discovery Foundation New York NY $ 313,357 Drug Discovery Research Fund Annual Conference of the Gay and American Association of Physicians for Human Rights San Francisco CA $ 50,000 Lesbian Medical Association American Cancer Society Austin TX 2008 Cattle Baron's Ball$ 5,000 American Cancer Society Inc. Hershey PA Relay For Life$ 5,000 American Cancer Society Inc. -

CVS Health Announces COVID-19 Resources for Aetna Members
CVS Health announces COVID-19 resources for Aetna members In response to the rapidly evolving COVID-19 outbreak, CVS Health announced a series of steps designed to support the health and well-being of Aetna members, ensure patient access to medication, and remove barriers to care. Effective immediately: Aetna will waive co-pays for all diagnostic testing related to COVID-19. This policy will cover the cost of physician-ordered testing for patients who meet CDC guidelines, which can be done in any approved laboratory location. Aetna will waive the member costs associated with diagnostic testing for all Commercial (including small group Aetna Funding Advantage, AFA), Medicare and Medicaid lines of business. Self-insured plan sponsors will be able to opt-out of this program at their discretion. For the next 90 days, Aetna will offer zero co-pay telemedicine visits for any reason. Aetna members should use telemedicine as their first line of defense in order to limit potential exposure in physician offices. Cost sharing will be waived for all virtual visits through Aetna-covered Teladoc® offerings and in-network providers delivering synchronous virtual care (live videoconferencing) for all Commercial plan designs, including small group Aetna Funding Advantage. Self-insured plan sponsors will be able to opt-out of this program at their discretion. CVS Pharmacy will waive charges for home delivery of prescription medications. With the Centers for Disease Control and Prevention encouraging people at higher risk for COVID-19 complications to stay at home as much as possible, this is a convenient option to avoid coming to the pharmacy for refills or new prescriptions. -

TC-017 Butterfield, Dawn (National Community Pharmacy Association)
Peter Mucchetti, Chief Healthcare and Consumer Products Section, Antitrust Division Department of Justice 450 Fifth Street NW, Suite 4100 Washington, DC 20530 December 13, 2018 Dear Judge Leon: Thank you so much for inviting or allowing comments on the proposed CVS/Aetna merger. We (pharmacy owners/pharmacists) were not happy when our own organization - NCPA - National Community Pharmacy Association was referenced in the Congressional Hearing on this merger earlier in the year that "we were fine" with the merger as expressed by Tom Moriarty - CVS General Counsel to the Congressional Committee. This most assuredly is NOT the case I can assure you. However that was communicated - and not clarified by our association is an issue we are all still highly upset about as I believe that was the time to publicly state our vehement opposition to this merger. In other words, a major ball was dropped and a bell was rung that couldn't be unrung. That being said - we, as business owners and professionals don't know how we can state our issues - which are numerous and we were being told by virtually everyone that the merger is a "done deal" as the FTC and DOJ didn't have an issue with it. I hope you receive communication from people who took the time to do so. Some people are frozen and/or feel defeated as they believe there's nothing to stop this train and have given up. I can say with certainty that for every letter from a pharmacist/pharmacy owner/pharmacy professor - there were 1000 that went along with it! I'm enclosing some material you may already have, but the infographic on the states shows to the extent of what can happen when your competition sets YOUR price. -

Carrier Codes
CARRIER CARRIER PHONE CODE CARRIER NAME NUMBER CARRIER ADDRESS CITY STATE ZIP CODE 326 1199 NATIONAL BENEFIT FUN (646) 473-7160 PO BOX 1034 NEW YORK NY 10108-1034 589 1199 SEIU NATIONAL BENEFI (888) 819-1199 330 WEST 42ND STREET NEW YORK NY 10036 28 AARP HEALTH ADVANTAGE HEA (800) 227-7789 PO BOX 740819 ATLANTA GA 30374-0819 D6K AARP MEDICARE COMPLETE PL PO BOX 66773 ST LOUIS MO 63166 D6P AARP MEDICARE COMPLETE PL (800) 393-0939 950 WINTER ST SUITE 3800 WALTHEM MA 02451 D8T AARP MEDICARE RX PREFERRE (888) 867-5575 PO BOX 29300 HOT SPRINGS AR 71903 D9H AARP MEDICARE RX PREFERRE 600 NEW LONDON AVE CRANSTON RI 02920 D9L AARP MEDICARE RX SAVER 600 NEW LONDON CRANSTON RI 02920 D8V AARP MEDICARERX SAVER (PD (888) 867-5575 PO BOX 29300 HOT SPRINGS AR 71903 D4Q ABRAZO ADVANTAGE 7878 NORTH 16TH STRE SUITE 105 PHOENIX AZ 85020 301 ACADIA INSURANCE CO. (888) 786-1170 PO BOX 9168 MARLBOROUGH MA 01752 390 ACE INTERNATIONAL INSURAN (800) 262-8028 PO BOX 15417 WILMINGTON DE 19850 470 ACE USA (860) 731-6800 PO BOX 5001 HARTFORD CT 06102-5001 30 ACORDIA BENEFIT SERVICES PO BOX 18197 COLUMBUS OH 43218 346 ACS HEALTH NET - NORTHEAS (800) 441-5741 PO BOX 14700 LEXINGTON KY 40512 768 ADMINISTRATION CONCEPTS ( (800) 226-5116 994 OLD EAGLE SCHOOL ST E1 WAYNE PA 19087 795 ADMINISTRATIVE CONCEPTS, (888) 293-9229 994 OLD EAGLE SCHOOL SUITE 1005 WAYNE PA 19087-1802 101 ADMINISTRATIVE ENTERPRISE (602) 789-1101 3404 WESTCHESTER DR PHOENIX AZ 85051-9588 267 ADMINISTRATIVE SERVICE CO (800) 634-8816 3301 E. -

Minuteclinic and Lifespan Agree to Clinical Collaboration in Rhode Island --Agreement Includes Integration of Electronic Medical Records
Media Contacts: Carolyn Castel Brent Burkhardt Gail Carvelli (401) 770-5717 (410) 986-1303 401-444-7299 CVS Health TBC (for MinuteClinic) Lifespan [email protected] [email protected] [email protected] MinuteClinic and Lifespan Agree to Clinical Collaboration in Rhode Island --Agreement Includes Integration of Electronic Medical Records-- Woonsocket, R.I. & Providence, R.I., Oct. 27, 2014 -- MinuteClinic, the retail medical clinic of CVS Health, and Lifespan, Rhode Island’s largest health system, which includes five partner hospitals and multiple physician groups, announced today that they have entered into a clinical collaboration agreement. The affiliation will enhance access to high quality, affordable health care services at MinuteClinic walk-in medical clinics opening inside select CVS/pharmacy stores in Rhode Island beginning later this week. The new Rhode Island clinics, opening this fall and in 2015, will be located in Cranston, East Greenwich, North Smithfield, Providence, Wakefield, Westerly and Woonsocket. The collaboration encourages interaction between Lifespan and MinuteClinic providers to improve coordination and access to care for patients seen at MinuteClinic locations. Toward that end, both organizations will integrate their electronic medical records to further promote exchange of clinical information, with patients’ permission. For those patients who do not have regular access to primary care, MinuteClinic provides assistance in finding a primary care physician. MinuteClinic locations in Rhode Island will be open seven days a week, offering weekday evening hours with no appointment necessary and most health insurance accepted. The clinics will be staffed by nurse practitioners and physician assistants who provide treatment for common family illnesses and administer wellness and prevention services, including health-condition monitoring for patients with chronic diseases. -

Overview of Ftc Antitrust Actions in Pharmaceutical Services and Products
OVERVIEW OF FTC ANTITRUST ACTIONS IN PHARMACEUTICAL SERVICES AND PRODUCTS Health Care Division Bureau of Competition Federal Trade Commission Washington D.C. 20580 Markus H. Meier Assistant Director Bradley S. Albert Deputy Assistant Director Saralisa C. Brau Deputy Assistant Director September 2009 TABLE OF CONTENTS Page I. INTRODUCTION. ........................................................... 1 II. CONDUCT INVOLVING PHARMACEUTICAL SERVICES AND PRODUCTS. 3 A. Monopolization. ...................................................... 3 B. Agreements Not to Compete. ............................................ 8 C. Agreements on Price or Price-Related Terms. 14 D. Agreements to Obstruct Innovative Forms of Health Care Delivery or Financing. 20 E. Illegal Tying and Other Arrangements. .................................... 20 III. PHARMACEUTICAL MERGERS. ........................................... 20 A. Horizontal Mergers Between Direct Competitors. 20 B. Potential Competition Mergers. ......................................... 44 C. Innovation Market Mergers. ............................................ 47 D. Vertical Mergers...................................................... 49 IV. INDUSTRY GUIDANCE STATEMENTS...................................... 50 A. Advisory Opinions. ................................................... 50 B. Citizen Petition to the Food and Drug Administration. 51 V. AMICUS BRIEFS. ......................................................... 51 VI. INDICES. ............................................................ -

Directions to Walgreens Closest to My Location
Directions To Walgreens Closest To My Location Tinglier Corky sometimes release his rockweed whencesoever and unburdens so vertically! Zollie still herborized deathy while manufactured Morty feeds that equivalent. Andonis is dispossessed: she backspacing austerely and stole her kaiserdom. Click continue to caring spirit of apple inc in no es responsable, family can i try passport applicants when used the content provided in my walgreens subsidiary that the features plenty of dissimilar metals which typically covered drugs safer and You can replicate search Walgreens 24 hour Pharmacy Near tree in Google Maps to get. Drive-Thru Testing Available by Appointment Snohomish. Please arrive in my walgreens locations offer all states that location for direct to encourage you can. The lenawee county program manager at walgreens customer service to target resource does not boil dry in. Members and walgreens locations only testing site is located in my results found for breaking news tip direct or an inference may take advantage of. The prophet time fix it operates is when ordinary water intake all out during the vaporizer. Error has issued a large corporation is your eligibility requirements here and operated by a launch pad to perform the closest location to walgreens my facility type of the final arbiter of. Us to my experience. Fees and limits apply. We are located in to drive to treat the closest location. List Close menu Find retail Store Prescriptions Back Prescriptions Refills Order Status Records Transfer of New Auto Refills Drug Info Getting. Each vaccination team being responsible for different own scheduling and paperwork. American nurses credentialing center is to my results to proceed, locations or location and. -

Convenience Care Centers
10/15/2019 7:16:21 PM Page 1 of 2 Find out more about the doctors and services listed in the Cigna directory and read important notices and disclosures for your state, https://hcpdirectory.cigna.com/assets/docs/hcpdirectory/disclaimer.html? WT.mc_id=PDF_DIR_Disclaimer&WT.z_dir_cc=HDC001 Convenience Care Minuteclinic Minuteclinic Minuteclinic Clinic 10101 W Flagler St 306 Lincoln Rd 5800 S University Dr Miami , FL 33174 Miami Beach , FL 33139 Davie , FL 33328 Minuteclinic (866) 389-2727 (866) 389-2727 (866) 389-2727 12955 SW 112th St Miami , FL 33186 Participates in: LocalPlus, Participates in: LocalPlus, Participates in: LocalPlus, (866) 389-2727 OAP, OAPC, PPO, HMO OAP, OAPC, PPO, HMO OAP, OAPC, PPO, HMO Participates in: LocalPlus, Minuteclinic Inside Target Minuteclinic Minuteclinic Inside Target OAP, OAPC, PPO, HMO 10101 W Flagler St 12401 Miramar Pky 5800 S University Dr Miami , FL 33174 Miramar , FL 33027 Davie , FL 33328 Minuteclinic (866) 389-2727 (866) 389-2727 (866) 389-2727 7800 SW 104th St Miami , FL 33156 Participates in: LocalPlus, Participates in: LocalPlus, Participates in: LocalPlus, (866) 389-2727 OAP, OAPC, PPO, HMO OAP, OAPC, PPO, HMO OAP, OAPC, PPO, HMO Participates in: LocalPlus, Minuteclinic Minuteclinic Minuteclinic OAP, OAPC, PPO, HMO 10701 NW 41st St 4701 S Flamingo Rd 4610 S University Dr Doral , FL 33178 Cooper City , FL 33330 Davie , FL 33328 Minuteclinic Inside Target (866) 389-2727 (866) 389-2727 (866) 389-2727 7800 SW 104th St Miami , FL 33156 Participates in: LocalPlus, Participates in: LocalPlus, Participates -

PRECEDENTIAL UNITED STATES COURT of APPEALS for the THIRD CIRCUIT ___Nos. 14-4202, 14-4203, 14-4204, 14-4205, 14-4206, 14-46
Case: 14-4203 Document: 003112598145 Page: 1 Date Filed: 04/19/2017 PRECEDENTIAL UNITED STATES COURT OF APPEALS FOR THE THIRD CIRCUIT ______ Nos. 14-4202, 14-4203, 14-4204, 14-4205, 14-4206, 14-4602 & 14-4632 ______ IN RE: LIPITOR ANTITRUST LITIGATION Rite Aid Corporation; Rite Aid Hdqtrs. Corporation; JC (PJC) USA, LLC; Maxi Drug, Inc. d/b/a Brooks Pharmacy; Eckerd Corporation, Appellants in No. 14-4202 Walgreen Company; The Kroger Co.; Safeway, Inc.; Supervalu, Inc.; HEB Grocery Company L.P., Appellants in No. 14-4203 Giant Eagle, Inc., Appellant in No. 14-4204 Meijer, Inc.; Meijer Distribution, Inc., Appellants in No. 14-4205 Rochester Drug Co-Operative, Inc.; Stephen L. LaFrance Pharmacy, Inc. d/b/a SAJ Distributors; Burlington Drug Company, Inc.; Value Drug Company; Professional Drug Company, Inc.; American Sales Company LLC, Appellants in No. 14-4206 Case: 14-4203 Document: 003112598145 Page: 2 Date Filed: 04/19/2017 A.F.L.-A.G.C. Building Trades Welfare Plan; Mayor and City Council of Baltimore, Maryland; New Mexico United Food and Commercial Workers Union’s and Employers’ Health and Welfare Trust Fund; Louisiana Health Service Indemnity Company, d/b/a Blue Cross/Blue Shield of Louisiana; Bakers Local 433 Health Fund; Twin Cities Bakery Workers Health and Welfare Fund; Fraternal Order of Police, Fort Lauderdale Lodge 31, Insurance Trust Fund; International Brotherhood of Electrical Workers Local 98; New York Hotel Trades Counsel & Hotel Association of New York City, Inc., Health Benefits Fund; Edward Czarnecki; Emilie Heinle; Frank Palter; Andrew Livezey; Edward Ellenson; Jean Ellyne Dougan; Nancy Billington, on behalf of themselves and all others similarly situated, Appellants in No. -

CVS Corporation 2004 Annual Report H Cvrs Narrative 3/17/05 12:18 PM Page +6
h_cvrs_narrative 3/17/05 12:17 PM Page +3 At CVS, it’s all in a day’s work... CVS Corporation 2004 Annual Report h_cvrs_narrative 3/17/05 12:18 PM Page +6 2:00am Another customer finds relief. From a toddler’s ear infection to a flare up of asthma, illnesses often have little regard for the clock. CVS has been doing its part to help, with approximately 60 percent of our stores offering 24- or extended- hour service. It’s just one of the many ways we make our pharmacy experience “CVS easy.”™ After all, the pharmacy accounts for 70 percent of our overall sales. CVS has a 13.5 percent share of the retail pharmacy market nationally, and we fill one in every five prescriptions in markets where we operate. Thanks to the Pharmacy Service Initiative (PSI) we launched in 2003, CVS is also making sure that prescriptions are “ready when promised”™ at any hour. Our pharmacy initiatives have freed up our pharmacists to spend more time counseling customers as well. As a result, customer service scores posted healthy gains in 2004. To realize similar benefits in the acquired Eckerd stores, we completed the training of more than 11,000 pharmacists and pharmacy support personnel on CVS systems, service processes, and standards. With more than 5,300 retail stores throughout the country and more on the way, CVS is well positioned to benefit from long-term pharmacy growth trends. The U.S. population is aging, healthcare costs are rising, and pharmaceuticals remain often the most cost-effective form of treatment. -

CVS Health Code of Conduct 1 CVS Health Code of Conduct 2 What We Stand For
Code of Conduct Dear CVS Health® Colleagues Over the years CVS Health has built an outstanding reputation with our customers, colleagues and key stakeholders. Our reputation for superior customer service and excellence in execution, coupled with our high level of integrity and sound business practices have helped us build a solid foundation of trust. This foundation is a valuable asset that has taken years to build, and is vital to our long-term success. As we look toward the future, we remain steadfast in our commitment to doing the right things in the right way, complying with laws and regulations and never compromising our standards. As you go about your day-to-day work and deal with challenging issues, I encourage you to refer to our Code. The Code was designed to help establish appropriate “rules of the road” for colleagues looking for the right solutions to ethical questions or issues and in obtaining additional guidance when the correct path is not clear. Each of your decisions and actions shape our reputation at CVS Health. That is why we must all commit to act with integrity while meeting our responsibilities. The Code is an excellent guide to doing the right thing, but it is not a substitute for good judgment, nor can it address every issue. So where there is no written rule or precedent, decisions need to be consistent with our company’s Purpose, Strategy and Values, which represent our guiding principles as an organization. In doing so we will continue to earn the trust that our stakeholders have placed in us. -

CVS Pharmacy Network
Participating Retail Pharmacies The following list shows the major chain pharmacies and affiliated groups of independent community pharmacies that accept your prescription benefit ID card. In addition to these, most independent pharmacies nationwide also take part in your prescription program. To find out if a pharmacy not listed here accepts your card, call the pharmacy directly. A C (continued) G (continued) A & P Pharmacy CVS Caremark Specialty Pharmacy Giant Eagle Pharmacy AAP / United Drugs CVS/Longs Giant Pharmacy Accredo Health Group, Inc. CVS/pharmacy Good Neighbor Pharmacy ACME Pharmacy Albertson’s Pharmacy American Pharmacy Cooperative / D H American Pharmacy Network Solutions Dahl’s Pharmacy Haggen Pharmacy American Home Patient Dierbergs Pharmacy Hannaford Food & Drug American Pharmacy Dillon Pharmacy Happy Harry’s Ameridrug Pharmacy Discount Drug Mart Harmons Pharmacy Apria Healthcare, Inc. Doc’s Drugs Harps Pharmacy Doctor’s Choice Pharmacy Aurora Pharmacy Harris Teeter Pharmacy Dominick’s Pharmacy Harvard Drug Drug Town Pharmacy Harvard Vanguard Medical Association B Drug Warehouse Harveys Supermarket Pharmacy Baker’s Pharmacy Drug World H-E-B Pharmacy Bartell Drugs Drugs for Less Health Mart Basha’s United Drug Duane Reade HealthPartners Bel Air Pharmacy Duluth Clinic Hen House Pharmacy Bi-Lo Pharmacy Henry Ford Pharmacy Bi-Mart Pharmacy Hi-School Pharmacies Biggs Pharmacy E Hilander Pharmacy Bioscrip Pharmacy EPIC Homeland Pharmacy Bloom Pharmacy Eaton Apothecary Horton & Converse Brookshire Brothers Pharmacy Econofoods