Real-Time Release of Volatile and Non-Volatile Components from Chewing Gum Using a Mechanical Chewing Device a DISSERTATION SUBM
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Retention Indices for Frequently Reported Compounds of Plant Essential Oils
Retention Indices for Frequently Reported Compounds of Plant Essential Oils V. I. Babushok,a) P. J. Linstrom, and I. G. Zenkevichb) National Institute of Standards and Technology, Gaithersburg, Maryland 20899, USA (Received 1 August 2011; accepted 27 September 2011; published online 29 November 2011) Gas chromatographic retention indices were evaluated for 505 frequently reported plant essential oil components using a large retention index database. Retention data are presented for three types of commonly used stationary phases: dimethyl silicone (nonpolar), dimethyl sili- cone with 5% phenyl groups (slightly polar), and polyethylene glycol (polar) stationary phases. The evaluations are based on the treatment of multiple measurements with the number of data records ranging from about 5 to 800 per compound. Data analysis was limited to temperature programmed conditions. The data reported include the average and median values of retention index with standard deviations and confidence intervals. VC 2011 by the U.S. Secretary of Commerce on behalf of the United States. All rights reserved. [doi:10.1063/1.3653552] Key words: essential oils; gas chromatography; Kova´ts indices; linear indices; retention indices; identification; flavor; olfaction. CONTENTS 1. Introduction The practical applications of plant essential oils are very 1. Introduction................................ 1 diverse. They are used for the production of food, drugs, per- fumes, aromatherapy, and many other applications.1–4 The 2. Retention Indices ........................... 2 need for identification of essential oil components ranges 3. Retention Data Presentation and Discussion . 2 from product quality control to basic research. The identifi- 4. Summary.................................. 45 cation of unknown compounds remains a complex problem, in spite of great progress made in analytical techniques over 5. -

Effect of Enzymes on Strawberry Volatiles During Storage, at Different Ripeness
Effect of Enzymes on Strawberry Volatiles During Storage, at Different Ripeness Level, in Different Cultivars and During Eating Thesis Presented in Partial Fulfillment of the Requirements for the Degree Master of Science in the Graduate School of The Ohio State University By Gulsah Ozcan Graduate Program in Food Science and Technology The Ohio State University 2010 Thesis Committee: Sheryl Ann Barringer, Adviser W. James Harper John Litchfield 1 Copyright by Gülşah Özcan 2010 ii ABSTRACT Strawberry samples with enzyme activity and without enzyme activity (stannous chloride added) were measured for real time formation of lipoxygenase (LOX) derived aroma compounds after 5 min pureeing using selected ion flow tube mass spectrometry (SIFT-MS). The concentration of (Z)-3-hexenal and (E)-2-hexenal increased immediately after blending and gradually decreased over time while hexanal concentration increased for at least 5 min in ground strawberries. The formation of hexanal was slower than the formation of (Z)-3-hexenal and (E)-2-hexenal in the headspace of pureed strawberries. The concentration of LOX aldehydes and esters significantly increased during refrigerated storage. Damaging strawberries increased the concentration of LOX aldehydes but did not significantly affect the concentration of esters. The concentrations of many of the esters were strongly correlated to their corresponded acids and/or aldehydes. The concentration of LOX generated aldehydes decreased during ripening, while fruity esters increased. Different varieties had different aroma profiles and esters were the greatest percentage of the volatiles. The aroma release of some of the LOX derived aldehydes in the mouthspace in whole strawberries compared to chopped strawberries showed that these volatiles are formed in the mouth during chewing. -

Factors Affecting Extraction of Adsorbed Wine Volatile Compounds and Wood T Extractives from Used Oak Wood ⁎ Eduardo Coelho , José A
Food Chemistry 295 (2019) 156–164 Contents lists available at ScienceDirect Food Chemistry journal homepage: www.elsevier.com/locate/foodchem Factors affecting extraction of adsorbed wine volatile compounds and wood T extractives from used oak wood ⁎ Eduardo Coelho , José A. Teixeira, Lucília Domingues, Teresa Tavares, José M. Oliveira CEB – Centre of Biological Engineering, University of Minho, Campus Gualtar, 4710–057 Braga, Portugal ARTICLE INFO ABSTRACT Keywords: During ageing, wood adsorbs volatile compounds from beverages. However, chemical interactions involved in Wood sorption sorption still remain unclear, as well as wood capacity to transfer such compounds to subsequent matrices when Hydrophobic adsorption reused. Therefore, extractions were conducted from used wood manipulating variables such as ethanol con- Wine volatiles centration, contact temperature and pH, in order to determine their effect in the interaction and consequent Wood extractives recovery of wine volatiles from wood. Mathematical models were outlined, which demonstrated an exclusive Wood ageing effect of ethanol concentration on the extraction of wine volatiles adsorbed in wood, more prominentfor compounds of higher hydrophobicity. Thus adsorption of wine volatiles was shown to be based on hydrophobic interactions. Recovery of wood extractives was also modeled, confirming the known positive effect of ethanol and temperature on the overall extraction of characteristic wood compounds. When reused, wood transferred wine compounds to hydroalcoholic matrices, demonstrating -

Fermentation and Ester Taints
Fermentation and Ester Taints Anita Oberholster Introduction: Aroma Compounds • Grape‐derived –provide varietal distinction • Yeast and fermentation‐derived – Esters – Higher alcohols – Carbonyls – Volatile acids – Volatile phenols – Sulfur compounds What is and Esters? • Volatile molecule • Characteristic fruity and floral aromas • Esters are formed when an alcohol and acid react with each other • Few esters formed in grapes • Esters in wine ‐ two origins: – Enzymatic esterification during fermentation – Chemical esterification during long‐term storage Ester Formation • Esters can by formed enzymatically by both the plant and microbes • Microbes – Yeast (Non‐Saccharomyces and Saccharomyces yeast) – Lactic acid bacteria – Acetic acid bacteria • But mainly produced by yeast (through lipid and acetyl‐CoA metabolism) Ester Formation Alcohol function Keto acid‐Coenzyme A Ester Ester Classes • Two main groups – Ethyl esters – Acetate esters • Ethyl esters = EtOH + acid • Acetate esters = acetate (derivative of acetic acid) + EtOH or complex alcohol from amino acid metabolism Ester Classes • Acetate esters – Ethyl acetate (solvent‐like aroma) – Isoamyl acetate (banana aroma) – Isobutyl acetate (fruit aroma) – Phenyl ethyl acetate (roses, honey) • Ethyl esters – Ethyl hexanoate (aniseed, apple‐like) – Ethyl octanoate (sour apple aroma) Acetate Ester Formation • 2 Main factors influence acetate ester formation – Concentration of two substrates acetyl‐CoA and fusel alcohol – Activity of enzyme responsible for formation and break down reactions • Enzyme activity influenced by fermentation variables – Yeast – Composition of fermentation medium – Fermentation conditions Acetate/Ethyl Ester Formation – Fermentation composition and conditions • Total sugar content and optimal N2 amount pos. influence • Amount of unsaturated fatty acids and O2 neg. influence • Ethyl ester formation – 1 Main factor • Conc. of precursors – Enzyme activity smaller role • Higher fermentation temp formation • C and N increase small effect Saerens et al. -

Concentrate Apple Juice Industry: Aroma and Pomace Valuation As Food Ingredients
Concentrate apple juice industry: aroma and pomace valuation as food ingredients Elisabete Coelho 1,*, Mariana Pinto 1, Rita Bastos 1, Marco Cruz 1, Cláudia Nunes 1,2, Sílvia M. Rocha 1 and Manuel A. Coimbra 1 1 LAQV-REQUIMTE, Department of Chemistry, University of Aveiro, 3810-193 Aveiro, Portugal.; [email protected], [email protected], [email protected], [email protected], [email protected], [email protected] 2 CICECO - Aveiro Institute of Materials, Department of Chemistry, University of Aveiro, Aveiro, 3810-193, Portugal; [email protected] * Correspondence: [email protected]; Tel.: +351 234 370706 (E.C.) Supplementary Material: Table S1. GC peak area of apple hydrodistillate obtained from the wet apple pomace and apple aroma resultant from the industrial juice processing, analysed by HS- SPME/GC-MS, and divided in positive and negative aroma and chemical families, according to the heatmap of Figure 4. Chromatographic peak area (10-7) Apple pomace Compound Standard Apple aroma Standard hydrodistilllate Deviation (n=3) Deviation (n=3) Positive aroma Alcohols Butanol 0.3 0.1 10.0 1.8 1-Hexanol 13.2 4.5 176.1 12.1 Trans-2-hexenol 0.0 0.0 25.6 1.0 6-methyl-5-hepten-2-ol 0.2 0.4 0.0 0.0 Aldehydes Acetaldeyde 0.1 0.2 1.6 0.2 Hexanal 4.5 4.0 41.6 3.8 Heptanal 0.4 0.6 0.0 0.0 Trans-2-hexenal 0.9 0.9 82.7 4.6 Octanal 0.3 0.5 0.0 0.0 Trans-2-heptenal 2.4 1.7 0.0 0.0 Nonanal 2.1 2.8 0.0 0.0 Benzaldehyde 41.0 15.8 2.2 0.4 Esters Methyl Acetate 1.7 1.1 0.0 0.0 Ethyl Acetate 5.5 3.2 32.3 2.9 Ethyl propionate 0.0 0.0 3.4 0.5 Propyl acetate 0.0 0.0 3.2 0.5 Methyl butanoate 0.0 0.0 -

Differences in Volatile Ester Composition Between Fragaria Ananassa and F
Scientia Horticulturae 150 (2013) 47–53 Contents lists available at SciVerse ScienceDirect Scientia Horticulturae journa l homepage: www.elsevier.com/locate/scihorti × Differences in volatile ester composition between Fragaria ananassa and F. ଝ vesca and implications for strawberry aroma patterns a,b b c b a,∗ Jing Dong , Yuntao Zhang , Xiaowei Tang , Wanmei Jin , Zhenhai Han a Institute for Horticultural Plants, College of Agronomy and Biotechnology, China Agricultural University, No. 2 Yuanmingyuan West Road, Haidian District, Beijing 100193, PR China b Institute of Forestry and Pomology, Beijing Academy of Agriculture and Forestry Sciences, No. 12 Ruiwangfen, Xiangshan, Haidian District, Beijing 100093, PR China c Beijing Vegetable Research Center, Beijing Academy of Agriculture and Forestry Sciences, Banjin, Haidian District, Beijing 100097, PR China a r t i c l e i n f o a b s t r a c t Article history: Esters are a very important component of strawberry (Fragaria sp.) aroma. In this study, fruit volatiles Received 8 April 2012 were isolated by solid-phase microextraction and analyzed by gas chromatography–mass spectrometry Received in revised form 1 November 2012 (SPME/GC–MS). It was found that F. × ananassa had more esters than F. vesca, but there was no significant Accepted 1 November 2012 difference in average relative content of esters. Twenty five esters were selected as predominant esters, 16 of which were prevalent in F. × ananassa and another 16 in F. vesca. Among them, hexyl acetate, Keywords: octyl acetate, ethyl butyrate, ethyl hexanoate, ethyl octanoate, methyl decanoate, and ethyl decanoate Volatile ester composition were found in both F. -

Comprehensive Mapping of Volatile Organic Compounds in Fruits
International PhD Program in Biomolecular Sciences XXVII Cycle Comprehensive Mapping of Volatile Organic Compounds in Fruits Tutor Dr. Fulvio Mattivi Department of Food Quality and Nutrition, Fondazione Edmund Mach Advisor Prof. Vladimir Shulaev Department of Biological Sciences, University of North Texas Ph.D. Thesis of Manoj Shahaji Ghaste Department of Food Quality and Nutrition Fondazione Edmund Mach 2013-2014 This thesis is lovingly dedicated to my Mother. Her support, encouragement, belief and constant love have sustained me throughout my life. Declaration I, Manoj Shahaji Ghaste confirm that this is my own work and the use of all material from other sources has been properly and fully acknowledged. Thesis abstract Volatile organic compounds (VOCs) are the key aroma producers in fruits and sensory quality of fruits is widely determined by qualitative and quantitative composition of VOCs. The aroma of grape is a complex of hundreds of VOCs belonging to different chemical classes like alcohols, esters, acids, terpenes, aldehydes, furanones, pyrazines, isoprenoids and many more. VOCs play important role as they determine the flavor of grapes and wine made from it. The objective of this thesis is to study of VOCs through development of different mass spectrometry based analytical methodologies and its applications for the comprehensive investigation and construction of database of the VOCs in grapes. First part of the study was dedicated to generation of a database of grape VOCs through the screening of multiple grape varieties (n=124) representing different species, color and origin. The experiment was carried out using headspace solid-phase microextraction (HS-SPME) and gas chromatography mass spectrometry (GC-MS) based approach and according to metabolomics protocols. -

Characterization of Key Aroma Compounds in Xiaoqu Liquor and Their Contributions to the Sensory Flavor
beverages Article Characterization of Key Aroma Compounds in Xiaoqu Liquor and Their Contributions to the Sensory Flavor Zhe Wang 1,2, Xizhen Sun 2, Yuancai Liu 2 and Hong Yang 1,* 1 College of Food Science and Technology, Huazhong Agricultural University, Wuhan 430070, China; [email protected] 2 Hubei Provincial Key Lab for Quality and Safety of Traditional Chinese Medicine Health Food, Jing Brand Research Institute, Jing Brand Co., Ltd., Daye 435100, China; [email protected] (X.S.); [email protected] (Y.L.) * Correspondence: [email protected]; Tel.: +86-027-8728-2111 Received: 25 May 2020; Accepted: 27 June 2020; Published: 1 July 2020 Abstract: The objective of this study was to investigate the aroma profile and major flavor compounds in Xiaoqu liquor by a combination of sensory profiling, GC-O/GC-MS analysis, and SBSE-GC-MS. A total of 57 important volatile compounds (FD 2) were screened based on the retention ≥ indices, aromatic characteristics, standard comparison, and aroma extract dilution analysis (AEDA). By calculating the odor activity values (OAVs), 32 aroma active compounds were identified in Xiaoqu liquor. In the aroma recombination experiments, these active compounds were dissolved in 46% ethanol water at their natural concentrations, resulting in successful simulations of the typical aromas of Xiaoqu liquor, including fruity aroma, sweet aroma, and grain aroma. Omission experiments showed that the critical compounds that contribute to the characteristic flavor of Xiaoqu liquor include ethyl octanoate, acetaldehyde, 1,1-diethoxyethane, isoamyl acetate, ethyl acetate, valeric acid, 1-octen-3-ol, and ethyl isovalerate. The results provide some guidance for upgrading the fermentation and microbial strain in the production of Xiaoqu liquor. -

B REGULATION (EC) No 1334/2008 of the EUROPEAN
2008R1334 — EN — 13.05.2016 — 011.001 — 1 This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents ►B REGULATION (EC) No 1334/2008 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC (Text with EEA relevance) (OJ L 354, 31.12.2008, p. 34) Amended by: Official Journal No page date ►M1 Commission Implementing Regulation (EU) No 872/2012 of 1 October L 267 1 2.10.2012 2012 ►M2 Commission Regulation (EU) No 545/2013 of 14 June 2013 L 163 15 15.6.2013 ►M3 Commission Regulation (EU) No 985/2013 of 14 October 2013 L 273 18 15.10.2013 ►M4 Commission Regulation (EU) No 246/2014 of 13 March 2014 L 74 58 14.3.2014 ►M5 Commission Regulation (EU) No 1098/2014 of 17 October 2014 L 300 41 18.10.2014 ►M6 Commission Regulation (EU) 2015/648 of 24 April 2015 L 107 15 25.4.2015 ►M7 Commission Regulation (EU) 2015/1102 of 8 July 2015 L 181 54 9.7.2015 ►M8 Commission Regulation (EU) 2015/1760 of 1 October 2015 L 257 27 2.10.2015 ►M9 Commission Regulation (EU) 2016/54 of 19 January 2016 L 13 40 20.1.2016 ►M10 Commission Regulation (EU) 2016/55 of 19 January 2016 L 13 43 20.1.2016 ►M11 Commission Regulation (EU) 2016/178 of 10 February 2016 L 35 6 11.2.2016 ►M12 Commission Regulation (EU) 2016/637 of 22 April 2016 L 108 24 23.4.2016 2008R1334 — EN — 13.05.2016 — 011.001 -
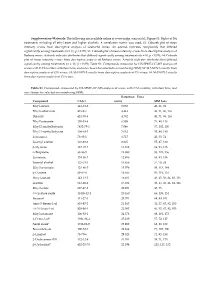
Supplementary File 1
Supplementary Materials: The following are available online at www.mdpi.com/xxx/s1, Figure S1: Biplot of BA treatments including all ethyl ester and higher alcohols. A correlation matrix was used; S2: Cobweb plot of mean intensity scores from descriptive analysis of Grenache wines. An asterisk indicates compounds that differed significantly among treatments (n = 11, p < 0.05); S3: Cobweb plot of mean intensity scores from descriptive analysis of Barbera wines. Asterisk indicates attributes that differed significantly among treatments (n = 10, p < 0.05); S4: Cobweb plot of mean intensity scores from descriptive analysis of Barbera wines. Asterisk indicates attributes that differed significantly among treatments (n = 10, p < 0.05); Table S1: Compounds measured by HS‐SPME‐GC‐MS analysis of wines with CAS number, retention time, and ions chosen for selected ion monitoring (SIM); S2: MANOVA results from descriptive analysis of GN wines; S3: MANOVA results from descriptive analysis of CS wines; S4: MANOVA results from descriptive analysis of CS wines. Table S1. Compounds measured by HS‐SPME‐GC‐MS analysis of wines with CAS number, retention time, and ions chosen for selected ion monitoring (SIM). Retention Time Compound CAS # (min) SIM Ions Ethyl acetate 141‐78‐6 3.053 43, 61, 88 Ethyl isobutyrate 97‐62‐1 4.443 43, 71, 86, 116 Diacetyl 431‐03‐8 4.782 43, 71, 86, 116 Ethyl butanoate 105‐54‐4 6.508 71, 88, 116 Ethyl 2‐methylbutyrate 7452‐79‐1 7.046 57, 102, 130 Ethyl 3‐methylbutyrate 108‐64‐5 7.632 55, 88, 130 Isobutanol 78‐83‐1 8.717 43, 55, -

Analysis of Odourant Compounds in Wine - with Headspace Solid-Phase Microextraction and Gas Chromatography- Mass Spectrometry
Analysis of odourant compounds in wine - With headspace solid-phase microextraction and gas chromatography- mass spectrometry By Emma Ödmar Analytical science program in chemistry with focus on forensics Candidate for Degree of Bachelor of Science School of Science and Technology Örebro university Spring term 2018 Table of contents Abstract .................................................................................................................................. 3 1. Introduction......................................................................................................................... 4 1.1 Aim ............................................................................................................................... 4 1.2 Scope ............................................................................................................................. 4 1.3 Background ................................................................................................................... 4 1.3.1 Wine ....................................................................................................................... 4 1.3.2 Headspace solid-phase microextraction (HS-SPME)................................................ 5 1.3.3 Gas chromatography-mass spectrometry (GC-MS) .................................................. 6 2. Materials and methods......................................................................................................... 7 2.1 Materials....................................................................................................................... -

This Document Is a Postprint Version of an Article Published in Food Chemistry © Elsevier After Peer Review. to Access The
This document is a postprint version of an article published in Food Chemistry © Elsevier after peer review. To access the final edited and published work see https://doi.org/10.1016/j.foodchem.2019.125555. Document downloaded from: Use of a flor yeast strain for the second fermentation of sparkling wines: effect of endogenous CO2 over-pressure on the volatilome Running Title: Use of a flor yeast strain for the diversification of sparkling wines by Rafael Martínez-Garcíaa, Yenifer Roldán-Romerob, Juan Morenoa*, Anna Puig-Pujolc, Juan Carlos Mauriciob and Teresa García-Martínezb a Department of Agricultural Chemistry, Marie Curie (C3) building, Agrifood Campus of International Excellence CeiA3, University of Córdoba, Ctra. N-IV-A, km 396, 14014 Cordoba, Spain b Department of Microbiology, Severo Ochoa (C6) building, Agrifood Campus of International Excellence CeiA3, University of Cordoba, Ctra. N-IV-A, kmm 396, 14014 Cordoba, Spain. cInstitut de Recerca i Tecnologia Agroalimentaries - Institut Català de la Vinya i el Vi). Plaça Àgora, 2. 08720 Vilafranca del Penedès (Barcelona), España. *Corresponding author E-mail address: [email protected] (J. Moreno). 1 ABSTRACT Saccharomyces cerevisiae flor yeast is used for the first time in sparkling wine-making. Twenty- six oenological variables and fifty-three volatile metabolites are quantified in the middle (P = 3 bar) and at the end (P = 6 bar) of the second fermentation, carried out in open and closed bottles. A heat- map of volatiles and the fingerprints obtained for ten chemical families and ten odorant series visualize the changes for each condition. Terpenes, fatty acids and volatile phenols increased their contents by pressure effect at the end of the study by 25.0, 7.8 and 2.2 %, respectively.